The Agent
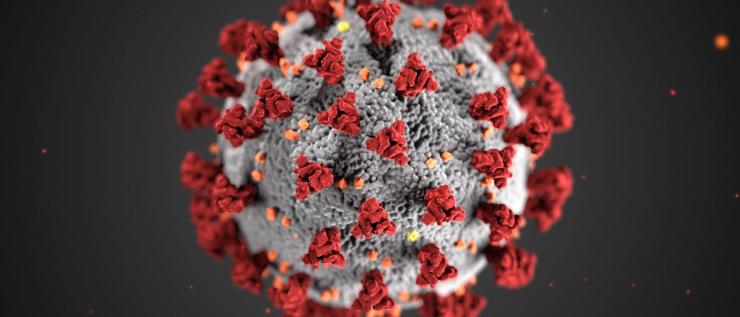
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus that causes the disease known as coronavirus disease 2019. The name of the disease is abbreviated COVID-19 where “CO” stands for corona, “VI” for virus, D” for disease, and “19” for the year in which it was identified. It was originally referred to as 2019 novel coronavirus. This virus, that is responsible for the current worldwide pandemic, is often referred to simply as coronavirus.
SARS-CoV-2 is in fact only one member of a large group of viruses known as coronaviruses. They are so named for their spiky or crown-like (corona) appearance under the microscope. Viruses within this family are genetically related, albeit distinct, viruses. They cause respiratory diseases in humans, some mild, such as the common cold, and some more severe. Other members of the coronavirus family can infect and cause a variety of diseases in several animal species.
In the two decades prior to 2019, two coronaviruses emerged that were capable of causing severe respiratory infection in humans. SARS-CoV emerged in late 2002 in Guangdong Province, China and caused Severe Acute Respiratory Syndrome (SARS). Another coronavirus, MERS-CoV, which caused Middle East Respiratory Syndrome (MERS), emerged in the Middle East in 2012. Unlike SARS-CoV-2, neither of these viruses produced a widespread, prolonged outbreak. This is because individuals infected by the SARS or MERS virus transmitted infection to others only after symptoms were apparent (whereas SARS-CoV-2 can spread even in the absence of symptoms). This enabled the quarantine of infected individuals and thereby prevented spread of infection of SARS and MERS.
SARS-CoV-2 emerged near the end of 2019 in Wuhan, China. It is not known precisely how the first humans were infected with SARS-CoV-2, but all of the available evidence points to an origin in nature. The virus is closely related to coronaviruses found naturally in bats. It is thought that, most likely, the virus passed from bats to an intermediate animal that came into close contact with humans who were then infected by this animal. At some point, the virus must have adapted to allow it to transmit readily from person to person (animal viruses generally do not spread easily between humans without adaptation). Zoonotic transmission has prompted multiple previous outbreaks, including those caused by influenza and HIV. Zoonosis plays a major role in the emergence of diseases new to humans.
Disease Transmission and Symptoms
SARS-CoV-2 can spread very easily from person to person. It appears to be transmitted more efficiently than influenza but not as efficiently as measles, one of the most contagious viruses known.
People infected with SARS-CoV-2 release virus particles from their mouth and nose when they cough, sneeze, speak, sing, or breathe heavily. The virus is contained within a range of liquid particles, from larger-sized respiratory droplets to smaller-sized aerosols. The larger respiratory droplets will fall out of the air more rapidly due to gravity, but the smaller particles can linger longer and spread further in the air. The concentration of virus particles decreases with distance.
COVID-19 is usually transmitted when respiratory droplets are inhaled or deposited on mucous membranes of the mouth or nose of an uninfected individual who is in close physical proximity (within about six feet) to an infected person.
Under some conditions, especially in enclosed, poorly ventilated spaces, a person may become infected through smaller aerosol droplets, and there is evidence that this type of transmission can occur over distances greater than six feet.
A third possible route of transmission can occur when a person touches surfaces that are contaminated with the virus (as a result of an infected person coughing, sneezing or touching a surface), and then touches their own nose, mouth, or eyes. Although this route was a concern early in the pandemic, further study has shown that is not a common means of transmission. Frequent hand washing, however, helps to minimize this risk.
It is possible for an infected person who does not display symptoms - or even know they are infected - to pass on the virus. The risk for transmission of SARS-CoV-2 is greatest in crowded, confined, and poorly ventilated spaces, such as some indoor bars and restaurants, particularly when people spend long periods of time in these locations. Spread is more likely to occur when an infected individual is not wearing a mask to restrict the release of virus particles in respiratory droplets and an uninfected person is not protected by a well-fitting mask.
Scientists refer to a reproduction rate, or R(t), to describe the rate at which a virus is spreading through the community. It is the average number of people who become infected by one infectious person. If the R(t) of a virus is 1, that means, on average, each infected person passes the virus to one other person. When the R(t) is greater than 1, then the virus is spreading at a faster rate, and when the R(t) is less than 1, then the spread of the virus is slowing.
The R(t) value varies during the course of an outbreak. It will be different at different times (it will be higher during a surge, for example) and at different locations (one state or country may be higher than another during the same time period).
The R(t) value depends in part of the behavior of the community. Mask wearing and physical distancing result in lowered R(t) values, indicating a slowing in the spread of the virus. The R(t) value is also affected by the level of immunity to the infectious agent within the community. When a large proportion of the population gains immunity, the R(t) value declines.
A wide variety of symptoms, ranging from mild disease to severe illness, have been reported in people infected with SARS-CoV-2. COVID-19 symptoms include fever and chills, cough, shortness of breath or difficulty breathing, fatigue, aches, and loss of taste or smell, among others. Symptoms usually appear 2 to 14 days after exposure to the virus. The risk for developing more serious complications from COVID-19 illness is generally higher in older adults and people who have underlying medical conditions, such as heart or lung disease, diabetes, obesity, or several other chronic health conditions.
While most people recover in a few days, some patients – referred to as long haulers - experience symptoms that linger for weeks or even months following the initial stage of infection. Symptoms may include fatigue, shortness of breath, headache, “brain fog”, and more serious complications that can affect the heart, lungs, or cause neurological issues. Persistent symptoms have been reported even in patients who exhibited only mild illness to begin with.
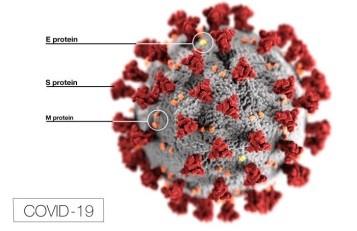
Virus Classification and Structure
The family of viruses known as the Coronaviridae is subdivided into four groups: alpha, beta, gamma, and delta coronaviruses. Members of the alpha and beta coronaviruses infect mammals, while the members of the gamma and delta groups primarily infect birds. Of the seven coronaviruses that are known to infect humans (all alpha or beta viruses), four cause mild upper respiratory illness and, together, are associated with 10–30 percent of common cold cases. The three coronaviruses capable of causing severe disease, SARS-CoV-2, SARS-CoV, and MERS-CoV, fall into the beta group.
Viruses are described and classified based on a number of characteristics including the type of genetic material they carry (DNA or RNA) and whether or not the virus particle is enclosed in a lipid layer known as an envelope. The genetic information of a coronavirus virus particle is encoded on a 30,000 nucleotide strand of positive-sense RNA, making it one of the largest genomes among RNA viruses. Its genome is surrounded by an envelope.
Coronavirus particles contain four main structural proteins: N (nucleocapsid), S (spike), M (membrane), and E (envelope). The N protein coats the genome, while the S, M, and E proteins are embedded in the lipid envelope. It is the protrusion of the club-shaped S protein from the envelope that gives a coronavirus particle its distinctive corona-like appearance under an electron microscope. The S protein recognizes the receptor protein on host cells and is required for the virus particle to gain entry into a cell, a step that is necessary for initiating its replication. The S protein is the primary target of vaccines.
The Problem
After emerging in Wuhan, China in late 2019, SARS-CoV-2 began to spread around the world. On March 11, 2020, the WHO declared COVID-19 to be a pandemic as the global spread of the virus began to accelerate and case numbers rose sharply in Europe, the United States, and other regions. One year later, on March 11, 2021, the numbers of cases of people infected with SARS-CoV-2 stood at about 120 million worldwide. (For up-to-date numbers, view the WHO Coronavirus (COVID-19) Dashboard). The first COVID-19 death was recorded in China in January 2020, and by September of that year the global toll had reached one million deaths. By July 2021, the number of cases approached 200 million globally, and the total recorded number of deaths surpassed 4 million.
As of July 2021, the United States had recorded the highest number of COVID-19 cases (about 34 million) and deaths (over 600,000) in the world, followed by India, and then Brazil. The United States reached a half million deaths in February 2021 - not much more than a year after the virus first emerged. This number exceeds the combined total of Americans who died from World War I, World War II, and the Vietnam War.
Beyond the illnesses and death toll, the pandemic disrupted many facets of everyday life, and resulted in school and business closures, travel restrictions, job losses and economic hardships, stressed healthcare systems, shortages of essential items, unrelated illnesses and deaths due to avoidance of routine medical care, and mental health issues. Many places have already experienced two or more surges in virus infections, and residents have had to endure restrictions on their movements.
Variants
Like other viruses, when SARS-CoV-2 replicates it makes occasional errors in copying its genetic code. Most of these mutations are insignificant. However, some of these changes can affect how transmissible or deadly the virus is or how it interacts with the human immune system. When a virus replicates in many individuals, as it does in the case in a pandemic (particularly among the unvaccinated), it becomes likelier that a virus will accumulate mutations. A subgroup of viruses that contain the same set of specific mutations is known as a variant. In addition to their scientific names, variants have been assigned Greek letters for ease of discussion.
There are multiple variants of SARS-CoV-2 circulating globally and within the United States. Currently (as of July 2021), four of these are classified by the CDC as variants of concern: Alpha (or B.1.1.7; originally detected in the United Kingdom and first identified in the United States. in December 2020), Beta (or B.1.351; first identified in South Africa and first found in the United States in January 2021), Gamma (or P.1; detected in travelers from Brazil at an airport in Japan and first detected in the United States in January 2021), and Delta (B.1.617.2; initially identified in India and first found in the United States in March 2021). In early April 2021, the Beta variant had become the dominant strain circulating in the United States, but by summer it had been largely replaced by the highly transmissible Delta variant. As of late July, the Delta variant accounted for greater than 80 percent of cases in the United States.
The reason that these particular variants are worrisome is that they are associated with higher rates of transmission, they may cause more severe disease and higher rates of death, and/or they may reduce the effectiveness of current vaccines. The presence of variants raises the possibility of reinfection, so that a person having contracted one variant of the virus could later become infected with another variant. Further concerns are that the variants may evade detection by some diagnostic tests and that they may have decreased susceptibility to therapeutic agents.
There are additional variants that are being monitored and characterized, and more variants are expected to arise. Individuals who are unvaccinated are at especially high risk of infection and serious disease from these variants.
Drugs and Vaccines
There is currently no cure for COVID-19. Clinical care consists of infection prevention and control measures and supportive care, including the use of supplemental oxygen and mechanical ventilators in cases of severe disease.
There is a limited list of therapeutic agents - for which there is evidence to show that they are effective in treating COVID-19 - that is currently available to clinicians for conditional use in patients, depending on the severity of their disease symptoms. The U.S. Food and Drug Administration (FDA) has issued Emergency Use Authorizations for anti-SARS-CoV-2 monoclonal antibodies for the treatment of outpatients with mild to moderate COVID-19. Remdesivir, an antiviral agent, is the only drug that is approved by the FDA for the treatment of COVID-19. It is currently recommended for use in hospitalized patients who require supplemental oxygen but not those that require mechanical ventilation. Dexamethasone, a corticosteroid, is recommended for hospitalized patients who require supplemental oxygen and mechanical ventilation but not those with milder disease. Additional drugs are in development and being tested. Currently, however, all of the drugs are restricted in their recommended usage to a subset of COVID-19 patients.
To date, the FDA has approved three COVID-19 vaccines for emergency use – those from Pfizer-BioNTech, Moderna, and Johnson and Johnson (J&J). All three have been demonstrated to be safe and effective. Millions of people in the United States have received COVID-19 vaccines under the most intense safety monitoring in United States history without serious side effects. Additional vaccines, including ones from Novavax and AstraZeneca, are in clinical trials or awaiting FDA approval (the vaccine from AstraZeneca has been approved for use in other countries). Although a rare and severe type of blood clot has been potentially linked to the J&J and AstraZeneca vaccines (which are based on a similar technology), these cases are extremely rare - approximately one in a million for the J&J vaccine.
The vaccines are of different types. The Pfizer-BioNTech and Moderna vaccines are messenger RNA (mRNA) vaccines and the J&J is a viral vector vaccine. All three stimulate the body to produce the SARS-CoV-2 spike (S) protein and generate an immune response against this protein, but the vaccines differ in the form in which the genetic information for the S protein is introduced into the body. None of the vaccines can cause COVID-19 or modify cellular DNA.
Although the vaccines were developed and tested and determined to be safe and effective in protecting people from COVID-19 within about a year of the emergence of the virus, the production of a sufficient number of doses and the logistics of distribution of the vaccine to the world's population has been a challenge. Ethical decisions have had to be made as to how to prioritize who should receive the limited number of doses initially available. The emergence of SARS-CoV-2 variants raises the concern that some of the vaccines could be less effective against certain variants.
A serious problem is the hesitancy of a significant proportion of the population in receiving a vaccine due to concerns about safety, misinformation about the vaccine, and other reasons. This is problematic because for COVID-19 to be brought under control, the population must achieve herd immunity. Herd immunity is when a large enough portion of the community is immune to a virus (either through natural infection or vaccination) so that the virus can no longer spread easily from person to person. This goal is most readily achieved - and with far fewer deaths - through mass vaccination than through natural infection. When herd immunity has been reached, the community is protected. This protection extends even to those who are unable to receive the vaccine, such as newborns, those who are allergic to the vaccine, and people with certain medical conditions.
The percentage of individuals who need to be vaccinated to achieve herd immunity varies from virus to virus. It is not known exactly what percentage is needed to reach this threshold for COVID-19, but experts estimate that it is in the range of 70 to 90 percent. Therefore, if enough people refuse vaccination, herd immunity will not be reached, and it would be more difficult to control the virus. Some experts suspect that COVID-19 will continue to circulate, at lower levels, for the foreseeable future.
Future pandemics
New diseases will continue to emerge. Other coronaviruses are lurking in the wild, primarily in bats, and could again spill over into humans. Or, a different type of virus could emerge, such as another pandemic strain of influenza. Each agent brings its own challenges depending on the manner and ease with which it spreads, how serious an illness it causes, its death rate, and the fear that it provokes.
Hopefully, however, we can learn from the current pandemic that preparation, coordination, education, and attention to scientific evidence can limit the toll that a pandemic can exact. That the mRNA vaccines and viral vector vaccines approved for emergency use were developed in record time, and demonstrated to be safe and effective in preventing COVID-19, shows that this technology can be adapted to many emerging viruses once their genetic sequence is made available.
The Research
Vaccines
Vaccines work by helping the body to develop immunity to an infectious agent without having to get the illness. Different types of vaccines work in different ways to offer protection, but all types of vaccines provide the body with a supply of specific immune system cells (memory T-lymphocytes as well as B-lymphocytes) that will "remember" the infectious agent and act quickly to fight that agent if it is encountered again in the future.
Members of the Department of Molecular Virology and Microbiology (MVM) at Baylor College of Medicine have been actively working on developing and evaluating COVID-19 vaccines. The department houses a Vaccine Research Center and has a long history of vaccine research. The MVM center is one a nationwide network of nine Vaccine Treatment and Evaluation Units (VTEUs) funded by the National Institutes of Health (NIH). Baylor's VTEU is headed by Dr. Hana El Sahly.
The Baylor VTEU was selected as one of 99 sites to participate in the Phase 3 clinical trial for Moderna's investigational mRNA-1273 vaccine to evaluate the efficacy of the vaccine at preventing COVID-19 disease. Dr. El Sahly also served as a national co-principal investigator of the trial.
The Moderna vaccine (like the Pfizer-BioNTech vaccine) is an mRNA vaccine. Although vaccines using this platform have not been previously approved for use, scientists have been studying this vaccine type for many years. It is well suited as a pandemic response strategy due to its flexibility and efficiency in design and manufacturing. The vaccine consists of a protective lipid-nanoparticle encapsulating the mRNA that encodes for the SARS-CoV-2 spike (S) protein. The S protein was suggested as a suitable vaccine target from earlier work during the 2002 SARS outbreak. Once a person is inoculated, the mRNA inside immune cells manufactures the S protein and displays pieces of the protein on the surface (the mRNA itself is subsequently destroyed). The immune system recognizes the S protein pieces as "foreign" and mounts an immune response. As a result, protection against possible infection in the future is achieved without having to risk serious illness.
The phase 3 trial launched in July 2020 enrolled approximately 30,000 adult volunteers nationwide who had an appreciable risk of SARS-CoV-2 infection. They were randomly assigned to receive two doses of either the vaccine or a placebo 28 days apart. The study participants were monitored for adverse effects to determine the safety of the vaccine. Efficacy was assessed by determining the percentage of participants for which the vaccine prevented COVID-19 cases and, separately, severe COVID-19. The results showed 94.1 percent efficacy at preventing COVID-19 illness, including severe disease. Aside from short-lived reactions, most commonly pain at the site of injection, no safety concerns were identified.
The VTEU is also involved in testing the safety and efficacy of the Novavax COVID-19 vaccine candidate, NVX-CoV2373, a protein-based vaccine that includes the SARS-CoV-2 S protein. MVM's Dr. Jennifer Whitaker serves as a co-principle investigator of this study. The trial is expected to enroll approximately 30,000 participants 18 years of age and older across the country and in Mexico, with Baylor enrolling approximately 250 participants. Two of three participants will receive two doses, three weeks apart, of NVX-CoV2373, while the remaining one third will receive a saline solution injection as a placebo. Participants will be followed for 24 months post-vaccination to monitor their health and safety.
Although the Moderna and other vaccines have been approved for emergency use in the United States and other developed countries, due to challenges and limitations in the production and distribution of these vaccines, there is still an urgent need for an accessible and low-cost COVID-19 vaccine suitable for low- and middle-income countries. MVM collaborates with a team of scientists led by Drs. Maria Elena Bottazzi and Peter Hotez of Baylor's National School of Tropical Medicine and Texas Children's Center for Vaccine Development (Texas Children's CVD) who are working to address this issue.
Texas Children's CVD has a three-fold mission to:
- Develop and test new low-cost and effective vaccines against emerging and neglected tropical diseases
- Build capacity for vaccine development locally and with foreign nations; and
- Guide and influence vaccine policy and advocacy.
Therefore, Texas Children's CVD applied its 10-year experience in advancing SARS and MERS vaccines to rapidly develop and test a safe and effective COVID-19 vaccine suitable for production, testing, and use in low- and middle-income country settings.
The COVID-19 vaccine candidate that the Texas Children's CVD researchers developed is based on a fragment of the SARS-CoV-2 viral protein called the receptor binding domain (RBD), which is part of the spike protein that the virus uses to attach and infect human cells. The idea is that the vaccine would stimulate neutralizing antibodies which would block the attachment of the virus to its receptor on the host cell, thus preventing infection and COVID-19. Using a modified RBD to make the protein more stable, the scientists tested the COVID-19 vaccine candidate in preclinical testing using animal models. The results were promising and showed that the vaccine can elicit a strong neutralizing antibody response and protection of vaccinated animals against a challenge with SARS-CoV-2.
In May 2020, the Texas Children's CVD COVID-19 vaccine candidate was licensed to an industrial vaccine manufacturer in India, Biological E. Limited. The company has advanced manufacturing and clinical testing capabilities and has demonstrated that they can produce up to 1.2 billion doses of the recombinant vaccine antigen. Biological E has completed a phase 1/2 clinical trial in seven centers around India to evaluate the safety and ability of the vaccine candidate to induce an immune response. They have received approval to start the Phase 3 clinical trial to be conducted at 15 sites across India. The goal is to increase vaccine access in low- and middle-income countries.
Most recently, Texas Children's CVD designed a next-generation COVID-19 vaccine prototype to address variants of concern and, similar to the first-generation COVID-19 vaccine, has performed a technology transfer to Biological E for further clinical evaluation. Lastly, Texas Children's CVD is embarking on programs to identify and evaluate broad and universal coronavirus vaccine prototypes.
After a person has been infected or vaccinated, it is important to know how long the antibody response lasts. The laboratory of Dr. Jason Kimata, in collaboration with laboratories of Drs. Bottazzi, Hotez, and Pedro Piedra, is working to answer this question.
Dr. Kimata's group is using lentivirus particles (a type of retrovirus) which have been pseudotyped, or modified, to express the SARS-CoV-2 spike protein on the surface. These pseudovirions are used in infection assays to study the anti-S antibody responses of infected and vaccinated subjects and test for virus neutralization, the ability of antibodies to block the part of the S protein needed to enter its target host cells. The researchers are working to dissect and evaluate selected S protein mutations that reduce susceptibility to neutralizing antibodies induced by infection or vaccination.
These studies will provide insight into the duration of natural or vaccine-induced immunity and how the antibody response might differ following infection with SARS-CoV-2 variants.
Monitoring SARS-CoV-2 in the Community

Clearly, during a viral outbreak, it is vital to know who is infected and the prevalence of the virus in the community at a particular time. Therefore, in response to the COVID-19 pandemic, the laboratory of Dr. Pedro Piedra, with the support of Baylor College of Medicine, transformed its CLIA certified molecular diagnostic laboratory into a service laboratory for testing SARS-CoV-2 for Baylor and its affiliates. Their SARS-CoV-2 service laboratory, led by Drs. Vasanthi Avadhanula and Erin Nicholson, opened on March 18, 2020. Within about a year, they had tested over 30,000 respiratory samples collected through the Baylor McNair Campus testing facility. The results generated from the SARS-CoV-2 service laboratory are reported weekly in a SARS-CoV-2 Summary Report to Baylor.
In parallel with the diagnostic service laboratory efforts, the Baylor Alkek Center for Metagenomics and Microbiome Research (CMMR) and the Human Genome Sequencing Center (HGSC) have been jointly performing similar clinical SARS-CoV-2 qPCR testing for mobile sites and congregate living settings in the greater Houston community under a contract with Harris County Public Health (HCPH). As of April 2021, the team has performed approximately 135,000 diagnostic tests since they began in April 2020. A complete data management infrastructure has been built, enabling all phases from accession to reporting of results (to patients and public health agencies). The CMMR-HGSC testing has performed outreach to other testing sites, including the City of El Paso. This outreach has been enabled by a further important relationship with the University of Texas School of Public Health (UT-SPH) whose Dean, Dr. Eric Boerwinkle, also serves as the Associate Director of the Baylor HGSC. The CMMR-HGSC testing pipeline is supporting Dr. Boerwinkle and colleagues from UT-SPH and Baylor in a Harris County SARS-CoV-2 prevalence study targeting up to 500 samples per day.
In recognition of the fact that new strains (e.g. variants of concern) have arisen, the testing programs are now using DNA sequencing for analysis of select SARS-CoV-2 viruses. This effort is led by Dr. Richard Gibbs, director of the HGSC, and Dr. Joseph Petrosino, of MVM and director of the CMMR, and performed in collaboration with Dr. Piedra and members of his laboratory. Multiple sequencing strategies are being employed to rapidly identify existing and novel SARS-CoV-2 variants from positive samples throughout the region. This effort is funded by several mechanisms, including the NIH-funded Genomic Center for Infectious Diseases program. The availability of detailed genetic information on the circulating viruses is a powerful aid in understanding the transmission dynamics of the SARS-CoV-2 outbreak and informing outbreak control decisions.
As well as the diagnostic service laboratory, which provides information regarding current infections, the Piedra laboratory has developed serological assays, led by Drs. Xunyan Ye and Laura Angelo, to study antibody responses induced by SARS-CoV-2 infection and vaccines. These tests are important in gauging how many people may have been previously infected with the virus (perhaps unknowingly), as well as the effectiveness of vaccines in eliciting antibodies. Using these assays, the scientists are working with the City of Houston Health Department and Harris County Health Department to determine the seroprevalence, or percentage of individuals who have antibodies to SARS-CoV-2, in different segments of the community.
Active surveillance for SARS-CoV-2 in children is also being conducted by the Piedra laboratory along with Dr. Julie Boom from Texas Children's Hospital who leads a CDC sponsored Acute Respiratory Illness surveillance study known as the New Vaccine Surveillance Network.
During an outbreak, it is valuable to have real-time information about the prevalence of the disease and the dynamics of infection in the community. While diagnostic testing is highly beneficial, results can lag up to a couple of weeks behind the onset of infection, so scientists have turned to wastewater monitoring as a way to get an earlier warning of outbreaks. This approach can be applied to SARS-CoV-2 surveillance because although COVID-19 is primarily considered a respiratory disease, diarrhea is a common symptom and approximately 40 percent of people with COVID-19 shed the virus in their stools.
Drs. Anthony Maresso, Piedra, and their colleagues in MVM - in conjunction with the Houston Health Department and Rice University - have developed a system to detect SARS-CoV-2 RNA levels on a weekly basis at 39 distinct wastewater treatment plant sites serving over 3.6 million people in and around the Houston area. Viral RNA levels were determined simultaneously by two independent laboratories and followed weekly for 34 weeks, both before, during, and after the 2020 April and July surges in cases, and surveillance is ongoing. Their findings indicated that SARS-CoV-2 RNA wastewater levels are a strong predictive indicator of trends in the positivity rate two weeks in advance of diagnostic testing performed using nasal swabs. The scientists are currently making adjustments to enable tracking of viral variants in wastewater.
Wastewater surveillance is a simple and robust metric that serves as a low-cost, high-yield epidemiologic measure of the total SARS-CoV-2 viral load for assessment of outbreaks and the effectiveness of vaccines on the community level. This mechanism has the advantage of providing a population-based immediate indicator of viral load that does not depend on individuals self-reporting or presenting for testing. In contrast to nasal testing, which may capture only a snapshot of cases, wastewater surveillance offers the prospect of broad and comprehensive assessment of the total virome in a given community. Furthermore, individual wastewater lines from high-risk establishments such as nursing homes, prisons, and schools can be sampled. Such findings have been used in real-time to make public health interventions, including deployment of mobile testing teams or education to the community about the use of masks and distancing.
SARS-CoV-2 pathogenesis
As with any newly emergent virus, it is necessary to determine the mechanism of how the agent causes disease. A valuable tool for scientists is a model system in which they can study the pathogenesis of the virus. One such system that is particularly useful in the study of infectious diseases is organoids. Organoids are simplified and miniaturized versions of organs that mimic their three-dimensional structures. Organoids are easier to manipulate and can be used to study the interactions between the virus and the host cells it infects. They are also useful in the study of the effect of drugs on these interactions.
Towards this end, the laboratory of Dr. Piedra has developed expertise in the BSL-3 environment, and through the support of Drs. Mary Estes and Sarah Blutt, colleagues in MVM, they have developed airway organoids to perform translational and mechanistic studies on SARS-CoV-2 pathogenesis. These studies are being led by Dr. Anubama Rajan, a post-doctoral fellow, Dr. Avadhanula and others in Dr. Piedra's lab.
Public Service and Education
In addition to the ongoing research, MVM and affiliated faculty members have and continue to serve as expert commentators for a variety of local and national media outlets, with the goal of informing and advising the public about COVID-19, the vaccines, and other aspects of the outbreak. Drs. Robert Atmar, Maria Bottazzi, Hana El-Sahly, Peter Hotez, Anthony Maresso, Joseph Petrosino, Pedro Piedra, Catherine Troisi, and Jennifer Whitaker are among the faculty that have acted in this capacity.
One of the missions of the affiliated Texas Children's CVD is to advocate for sustained research funding for coronavirus and other tropical disease vaccine development and to combat vaccine hesitancy and anti-science movements in the United States and globally, and they serve as leaders in this global effort. Drs. Hotez, Bottazzi and many of the Center's faculty and staff actively participate in and lead special task forces, such as the Vaccine and Therapeutics of the Lancet COVID-19 Commission. In addition, they author and speak frequently on the topic.
SARS-CoV-2/COVID-19 Publications by MVM Faculty
Note: The information on this page was last updated July 26, 2021. Due the rapid accumulation of knowledge about SARS-CoV-2 and COVID-19, this page may not contain the most up-to-date information.
For More Information
General and health information about COVID-19
General information about COVID-19 (Centers for Disease Control and Prevention (CDC))
General information about COVID-19 (World Health Organization (WHO))
Dashboards showing numbers of cases and death and maps of COVID-19 distribution in the United States and worldwide
United States COVID-19 Cases and Deaths by State (CDC)
Global COVID-19 Cases and Death (WHO)
Map of United States COVID-19 Cases and Deaths (Johns Hopkins University)
Map of Global COVID-19 Cases and Deaths (Johns Hopkins University)
Drugs and vaccines
COVID-19 Treatment Guidelines (National Institutes of Health)
Information about COVID-19 Vaccines (CDC)
Description of Different COVID-19 Vaccines (CDC)
Facts about COVID-19 Vaccines (CDC)
COVID-19 Vaccinations in the United States (CDC)
Variants
SARS-CoV-2 Variant Classifications and Definitions (CDC)
SARS-CoV-2 Case Numbers (CDC)
Community Resources
Listing of National and Local Resources (BCM)







