
Arrhythmogenic right ventricular dysplasia/cardiomyopathy was first reported as the partial replacement of the right ventricular myocardium by fat or fibrous tissue. In 1965, researchers described the disease as "auricularization of the RV curve due to loss of the contractile power of the RV" (view reference). However, the description of the disease was later modified to "arrhythmogenic right ventricular dysplasia/cardiomyopathy." In 1996, ARVD/C was added to the WHO classification of cardiomyopathies.
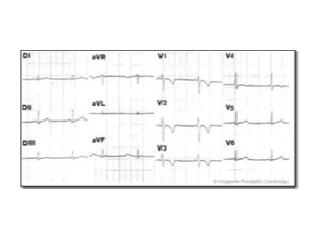
The name is derived from the observation that electrical disturbances of the heart (arrhythmogenic) are common and tend to affect the right ventricle more than the left ventricle. In addition, there is heart muscle disease (cardiomyopathy). The clinical course is characterized by ventricular arrhythmias (ventricular tachycardia), heart failure, syncope and sudden death.
ARVD/C is often patchy in its distribution, so abnormal areas may be surrounded by normal myocardium. If the heart is examined, the right side of the heart may appear to be thickened early in the disease, but later it may become dilated (enlarged) with a thinner wall. The muscle becomes reduced and instead there are thin layers of fat and fibrous tissue. Originally, it was thought that only the right side of the heart was affected, but it is now recognized that the left side of the heart can also be affected.
Frequency of ARVD/C
The true prevalence of ARVD/C is still to be determined, but it is likely to be an under recognized condition. In Italy, the prevalence has been reported as 1:5000 people, accounting for 20 percent of sudden deaths in young adults and 25 percent of cardiac sudden deaths among athletes. In one small study in the United States a frequency at autopsy of 0.55 percent among young adults with sudden cardiac death was reported.
Causes of ARVD/C
As yet, the causes of most cases of ARVD/C have not been identified. Familial cases of ARVD/C (due to the inheritance of a defective gene) account for at least 30 percent of cases, with autosomal dominant inheritance with reduced penetrance being the most common. In the remaining cases, it is believed to be due to an acquired etiology (e.g. virus infection) or to unidentified inheritance. The genes identified to date include the ryanodine receptor 2 (RYR2) and desmoplakin. A complex set of recessive disorders, Naxos disease (ARVD/C associated with palmoplantar keratoderma and woolly hair) and Carvajal syndrome (same as Naxos disease except affects LV), have been shown to be caused by homozygous mutations in plakoglobin and desmoplakin, respectively.
Viral Infection

The role of infectious agents in cases of ARVD/C has been proposed due to the common finding of inflammatory infiltrates in the myocardium, suggesting that ARVD/C is a sequela of myocarditis (like dilated cardiomyopathy). A number of viruses have been identified in the hearts of patients with ARVD/C, myocarditis, or DCM. In 1986, a virus called Coxsackievirus B was identified in the hearts of patients with either myocarditis or DCM. Subsequently, it has been shown that another virus, called adenovirus, is also commonly a cause of these conditions.
It has been suggested that when the virus infects the heart, it causes the body to react against the virus, much as the body's own defense system (immune system) normally attacks a virus infection, and this causes inflammation in the heart (myocarditis). Usually the virus will be eliminated, however in some patients the immune system may not totally eliminate the virus, and in these patients, the heart muscle continues to be damaged, leading to DCM. In some people, the body's immune system attacks and damages the heart even though the virus has been eliminated: this is termed auto immune disease. It has been shown that in some patients with ARVD/C, adenovirus and Coxsackievirus may be disease-causing.
We have recently reported a study looking for evidence of virus infection of the heart in 12 patients with non-familial ARVD/C. Enteroviruses (the virus family which includes the Coxsackieviruses) were identified in seven patients and adenovirus type 5 in another two patients.
Investigations of the role of viruses in the development of ARVD/C are ongoing in the Phoebe Willingham Muzzy Pediatric Molecular Cardiology Laboratory, while viral diagnosis is provided by the John Welsh Cardiovascular Diagnostic Laboratory.
Familial ARVD/C
All hereditary information is transmitted through DNA, which encodes many genes (approximately 100,000) that are transcribed to make specific proteins. Inherited disorders due to a single abnormal gene are transmitted to offspring in a predictable fashion, termed Mendelian transmission. As a result of gene mutations, abnormal genes located on any of the 22 autosomal pairs or the two sex chromosomes may produce phenotypes inherited by simple patterns classified as autosomal (dominant or recessive) or X-linked, respectively. When different genes induce the same phenotype, it is referred to as genetic heterogeneity, and most diseases in humans exhibit genetic heterogeneity.
Families with multiple individuals who have ARVD/C are likely to have a genetically inherited form. However, not all affected individuals have an affected parent because, in all autosomal dominant diseases, a certain proportion of cases occur due to a new mutation (i.e., they are sporadic). The parent whose germ cells contain the new mutation will be clinically normal, since the mutation affects only a single germ cell, but can transmit the disease-causing allele to their offspring.
The first gene for autosomal dominant ARVD/C was recently identified, encoding the cardiac ryanodine receptor 2 (RYR2) on chromosome 1q42-43. In addition, two genes responsible for Naxos disease and Carvajal syndrome, which are complex syndromes with an associated ARVD/C phenotype, have been identified as plakoglobin and desmoplakin, respectively. These genes encode proteins which are important in the cardiac adherens junctions. Recently, we have shown that desmoplakin mutations cause autosomal dominant ARVD/C as well.








