The Agent
Chikungunya is a disease that is caused by the chikungunya virus which is transmitted to humans by infected mosquitoes. Its name is derived from a word in the Makonde language that means contorted or bent, and it refers to the stooped appearance of its victims who suffer from severe joint pain.
Chikungunya (pronounced chik-un-GUN-ya) is similar to dengue virus in that it shares many of the same symptoms and is transmitted by the same species of mosquitoes.
The most common symptoms of chikungunya viral infection are fever and joint pain, often in the hands and feet. Other symptoms include muscle pain, headache, joint swelling, nausea, fatigue, and rash. Chikungunya is rarely a fatal disease, but the joint pain can be severe and debilitating. It typically lasts for a few days, although in some patients the pain can persist up to several weeks or even months.
Chikungunya is spread to people by two species of mosquitoes, Aedes aegypti (also known as yellow fever mosquito) and Aedes albopictus (also known as Asian tiger mosquito). Both mosquitoes are found in the southeastern United States and limited parts of the Southwest. Aedes albopictus has a wider range and is additionally found along the East Coast up through the Mid-Atlantic States and in the lower Midwest.
The mosquitoes become infected when they feed on a person infected with the chikungunya virus, and then the infected mosquitoes can spread the virus when they bite another person. These species of mosquitoes feed mostly during the daytime. Symptoms usually appear between 3 and 7 days following the bite of an infected mosquito, but can range from 2 to 12 days. The virus is not transmitted from person to person.
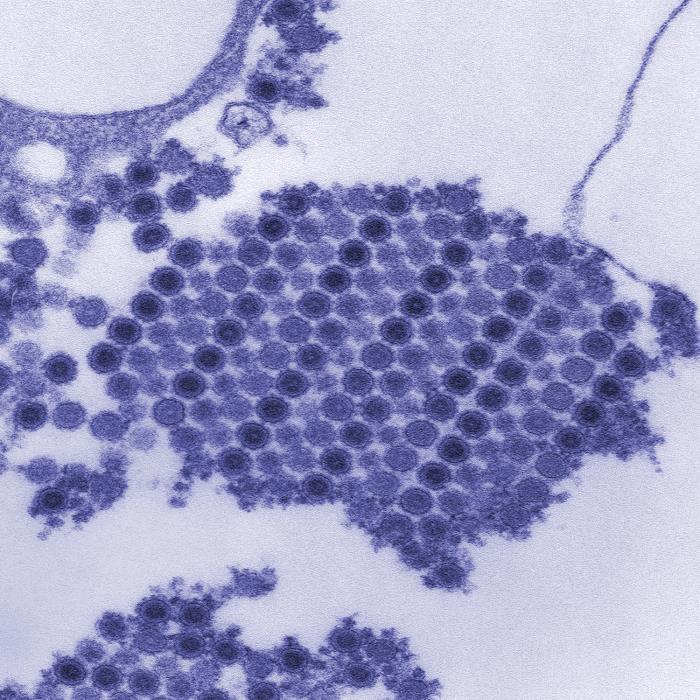
Chikungunya is classified as an alphavirus belonging to the Togaviridae family. The virus has a positive-sense, single-stranded RNA genome surrounded by a lipid envelope. The viral particles are small (60-70 nanometers in diameter) and spherical.
Outbreaks
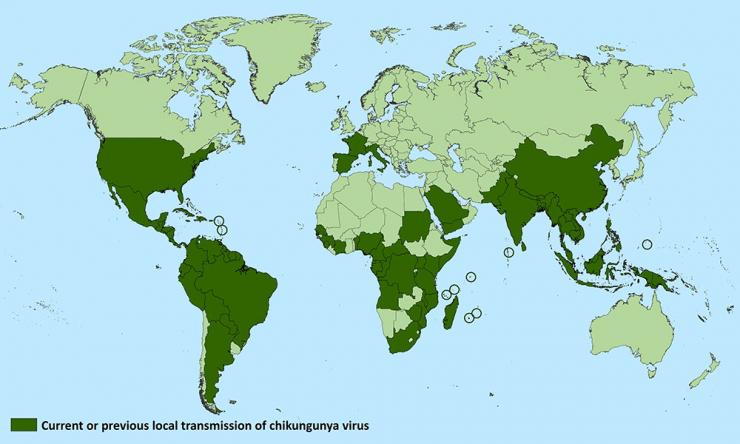
Chikungunya was first recognized in Tanzania in 1952, and human infections occurred in Africa at relatively low levels until around 2000 when there was a large outbreak in the Democratic Republic of Congo, followed by one in Gabon in 2007. Beginning in 2005, a major outbreak occurred in the islands of the Indian Ocean and countries in Southeast Asia. In 2007, chikungunya virus was documented for the first time in Europe when an outbreak involving nearly 200 individuals arose in Italy.
In December 2013, chikungunya emerged in the Americas, originating in St. Martin and then spread rapidly throughout other Caribbean islands. The infection was transmitted locally through infected mosquitoes found in the area. The outbreak expanded to Central America, South America, and North America. Since then, local transmission has been reported in 45 countries or territories in the Americas with more than 1.7 million cases suspected in these areas.
As of January 2016, there had been nearly 700 laboratory-confirmed cases of chikungunya virus infection from 44 states in the United States that were travel related. The highest number of cases were reported in California, followed by New York and Florida. There were some 200 locally acquired cases in U.S. territories, primarily Puerto Rico.
In July of 2014, the first locally acquired case of chikungunya virus was reported in Florida; in total there have been 12 cases originating in Florida. In June of 2016, it was announced that a person in Texas with no recent history of travel had tested positive for chikungunya virus.
Since that time, cases have continued to be reported (there were 114 chikungunya disease cases in the United States in 2017), but all of the most recent ones have occurred in travelers returning from affected areas.
The Problem
Since chikungunya virus is new to the Americas, most people are not immune to the virus. This means they can be infected and if bitten by another mosquito can introduce the virus into the local mosquito population.
There is no antiviral medicine to treat chikungunya viral infection. Treatment aims to alleviate symptoms through the use of pain medicine, fluids, and rest.
There is no licensed vaccine to prevent chikungunya infection. The best protection is to avoid mosquito bites in areas where the chikungunya virus is found by using insect repellents containing DEET and minimizing exposure to mosquitoes.
The types of mosquitoes that carry chikungunya virus are prevalent in many areas of the United States. As climate changes, the mosquitoes that transmit the virus may expand into additional regions. Infected mosquitoes are already present in Florida, and Texas health officials trapped a mosquito in the Houston area in the summer of 2014 that tested positive for the chikungunya virus.
The chikungunya virus has been spreading rapidly, and it is considered likely that it will continue to expand its range beyond the areas already affected. Although the risk of widespread transmission within the United States is considered low, given the fact that travel associated cases continue and that regions of the United States harbor the Aedes mosquitoes that transmit the virus, the possibility of some local transmission remains.
The Research
Diagnostic test for chikungunya virus
The laboratory of Dr. Rebecca Rico-Hesse has a very sensitive and rapid diagnostic tool available for detecting chikungunya virus in patient blood. They developed a quantitative RT-PCR test that detects and indicates approximately how many viral particles are in a febrile patient’s blood. This could possibly correlate with a prognosis, in terms of fever duration, possibility for chronic joint pain, and infectiousness to mosquitoes.
The diagnostic test can detect all Chikungunya virus variants (or “genotypes”, including the West African, Asian and ECSA genotype), and contains a control positive sample (to reduce false positives) that was prepared in a high containment facility at BCM. The protocol takes 2-4 hours, uses a very small amount of patient serum (100 microliters), and has been tested on a patient sample to confirm its sensitivity.
Dr. Rico-Hesse has applied for funding to develop this into a routine test available to clinicians treating febrile patients who might have acquired chikungunya in Texas. It would be a very valuable test, as it is expected that chikungunya virus will be transmitted by mosquitoes along the Gulf Coast in the not-too-distant future.
Effect of mosquito saliva alone on the human immune response
In another area of study, Rico-Hesse and coworkers, using a 'humanized mouse' model to study dengue virus infection, found that mosquito saliva alone - in the absence of any virus - can trigger a human immune response which may significantly affect disease development not only by dengue virus, but also by other Aedes mosquito-transmitted viruses including chikungunya virus and Zika virus.
The researchers used a model system that consisted of mice naturally born without their own immune system, but which had received human stem cells which could then give rise to many of the components of the human immune system, so factors involved in disease development could be studied. These animals were injected with dengue virus either through a mosquito bite or through needle injection.
They found that animals receiving the virus through a mosquito bite developed a more human-like disease with more of a rash, more fever and other characteristics that mimic the disease presentation in humans than did the needle-injected mice.
This led the scientists to investigate the role of mosquito saliva on disease development, hypothesizing that the mosquitoes may not just be acting like ‘syringes,’ merely injecting viruses into the animals they feed on, but rather that their saliva may contribute significantly to disease development.
To test this idea, virus-free mosquitoes were allowed to feed on the humanized mice, and then the researchers took blood and a number of other tissue samples six hours, 24 hours and seven days after the mosquitoes bit the mice. They determined the levels of cytokines, as well as the number and activity of different types of immune cells, using highly sensitive techniques and compared these results with those obtained from humanized mice that had not been bitten by mosquitoes.
They discovered that mosquito-delivered saliva induced a varied and complex immune response. Both the immune cell responses and the cytokine levels were affected. At various time points, the levels and activities of other types of immune cells also increased as others decreased.
Overall, the researchers found evidence that mosquito saliva alone can trigger long-lasting immune responses – up to seven days post-bite – in multiple tissue types, including blood, skin and bone marrow. The diversity and complexity of the immune response was a surprise to the investigators.
The researchers would now like to determine which of the more than 100 proteins in mosquito saliva are mediating the effects on the immune system and whether these effects could increase disease development. Identifying these proteins could help in the design of strategies to fight transmission of chikungunya, dengue, and other viral diseases transmitted by Aedes aegypti mosquitoes.
Glossary
Learn more about some of the technical terms found on our glossary of terms page.








 Credit
Credit