Hareem Hamza, MD (PGY2)
Celia Marginean, MD
View case images here.
A 53-year-old man with a history of type 2 diabetes mellitus presented with several weeks of subacute abdominal pain, unintentional weight loss, and poor oral intake.
Evaluation revealed acute bilateral pulmonary emboli and widely metastatic malignancy of unknown primary, with extensive hepatic and lung metastases, peritoneal carcinomatosis, and malignant ascites.
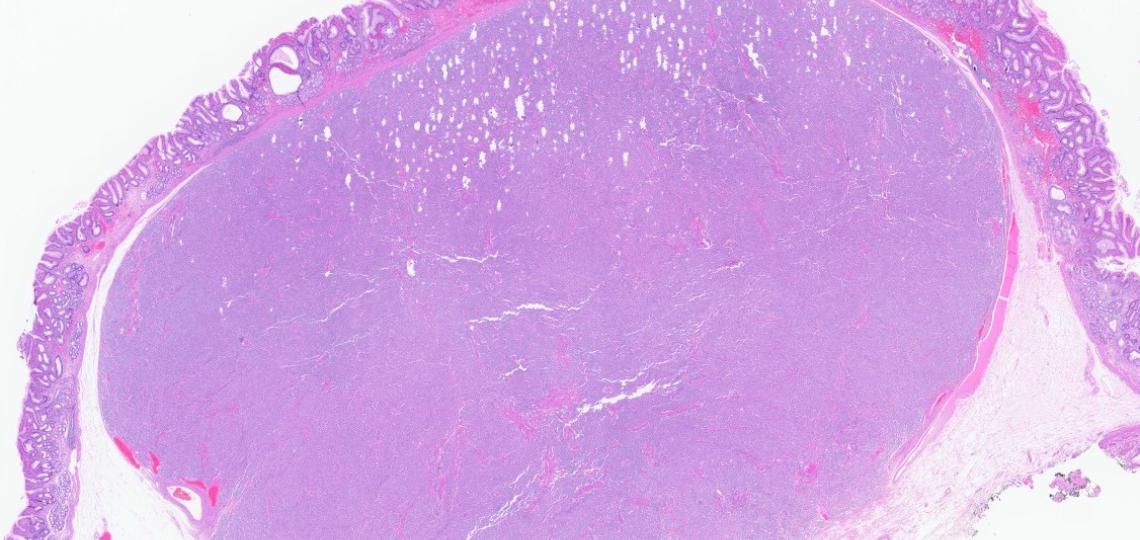
A biopsy of the liver was performed: see images in ppt.
Question: A liver mass biopsy shows adenocarcinoma with TTF-1 positivity in ~30% of tumor cells and CK-7 positivity. Imaging reveals a dominant gastric mass with nodal disease. Which of the following statements is MOST accurate regarding the interpretation of these findings?
A. Expression of TTF-1 is essentially specific for primary lung adenocarcinoma and excludes a gastrointestinal primary.
B. TTF-1 positivity with clone SPT24 is highly specific for lung and thyroid carcinomas and rarely occurs in other tumors.
C. Gastric adenocarcinomas can show focal TTF-1 positivity, particularly with clone SPT24, and metastatic deposits may retain this expression, potentially mimicking lung origin.
D. Clone 8G7G3/1 is more sensitive than SPT24 and would be expected to show stronger staining in gastric adenocarcinoma.
E. TTF-1 immunostaining alone is sufficient to determine the primary site in carcinoma of unknown origin.
Correct Answer: C.
Explanation: TTF-1 expression is clone-dependent. The SPT24 clone is more sensitive but less specific, detecting TTF-1 in a subset of non-pulmonary tumors, including gastric adenocarcinomas, where up to 25% of cases may show focal positivity.
Metastatic sites can retain this staining pattern, and some cases may also express Napsin A, creating a strong false impression of pulmonary origin.
In contrast, clone 8G7G3/1 is more specific but less sensitive. Therefore, TTF-1 (especially with SPT24) must be interpreted in the context of morphology, clinical findings, and a broader immunohistochemical panel, as reliance on this marker alone can lead to misclassification of tumor origin.
This case underscores two important diagnostic pitfalls.
First, TTF-1 expression, although classically associated with pulmonary and thyroid primaries, is not entirely specific and can be seen in a subset of gastric adenocarcinomas and other gastrointestinal malignancies, as well as occasionally in pancreatobiliary tumors such as cholangiocarcinoma. Therefore, reliance on a single immunohistochemical marker may lead to misclassification of the primary site. Accurate diagnosis requires integration of morphology, a comprehensive immunohistochemical panel, clinical history, and radiologic findings.
TTF-1 expression varies significantly among antibody clones, with clone 8G7G3/1 demonstrating higher specificity but lower sensitivity for lung adenocarcinoma compared with SPT24 and SP141. A subset of nonpulmonary tumors, particularly colorectal carcinomas, may also show TTF-1 positivity, especially with the latter clones. Therefore, TTF-1 expression alone cannot reliably distinguish primary lung cancer from pulmonary metastasis. Accurate diagnosis requires correlation with morphology, a broader immunohistochemical panel, and clinical findings.
Autoimmune metaplastic atrophic gastritis (AMAG)–associated gastric adenocarcinomas show significantly higher patchy TTF-1 and Napsin A immunoreactivity compared with adenocarcinoma not associated with atrophy. This staining may mimic metastatic lung adenocarcinoma, highlighting the need for careful interpretation (4).
Second, Metastatic disease can occasionally present in highly unusual locations. Tumor involvement within a rectal polyp or vascular lesion may mimic a primary colorectal neoplasm or even a benign process. Recognition of lymphovascular tumor emboli, along with correlation to a known primary malignancy, is essential to avoid diagnostic error. Careful attention to histologic features—such as the absence of surface dysplasia, evidence of mucosal cancerization, or prominent submucosal lymphangitic spread—should raise suspicion for metastasis. Accurate classification relies on judicious, targeted immunohistochemistry of limited biopsy material, interpreted within the context of clinical history, endoscopic findings, and imaging evidence of widespread disease.
References:
Choi, S. M., Furth, E. E., & Zhang, P. J. (2016). Unexpected TTF-1 Positivity in a Subset of Gastric Adenocarcinomas. Applied Immunohistochemistry & Molecular Morphology, 24(8), 603–607. https://doi.org/10.1097/PAI.0000000000000244
Noack, P., Grosse, C., Eschemann, S., Dislich, B., & Langer, R. (2025). Immunohistochemical TTF-1 and Napsin a Expression in Gastrointestinal Adenocarcinomas—Low Frequency but an Important Pitfall. Diagnostics (Basel), 15(12), 1490. https://doi.org/10.3390/diagnostics15121490
Vidarsdottir, H., Tran, L., Nodin, B., Jirström, K., Planck, M., Mattsson, J. S. M., Botling, J., Micke, P., Jönsson, P., & Brunnström, H. (2018). Comparison of Three Different TTF-1 Clones in Resected Primary Lung Cancer and Epithelial Pulmonary Metastases. American Journal of Clinical Pathology, 150(6), 533–544. https://doi.org/10.1093/ajcp/aqy083
Toussieng, T., Kozak, M., Burch, M., Gangi, A., Gong, J., Guindi, M., Lai, K. K., Hutchings, D. A., Larson, B. K., & Waters, K. M. (2026). Pulmonary immunohistochemical markers may be positive in gastric adenocarcinomas associated with autoimmune metaplastic atrophic gastritis. Histopathology, 88(3), 729–735. https://doi.org/10.1111/his.15526