About the Lab
The Gallo Lab is a highly interdisciplinary research group that aims to develop new treatments for brain cancers.
Our team consists of bioinformaticians, computational scientists, biochemists and molecular biologists. We use single-cell genomics, bulk genomic technologies, clinical samples and patient-derived preclinical models to identify epigenomic vulnerabilities in brain cancer. We focus on the epigenetic properties of brain tumor cells for several reasons.
First, because epigenetics determines cell function. In this context, we are especially interested in finding ways to block the epigenetic programs that enable brain tumor cells to behave similarly to stem cells.
Second, epigenetics is a strong driver in brain tumors, especially in children. We think epigenetic programs complement the few mutations in genes in pediatric brain cancer to initiate tumor formation, therapy resistance and recurrence. By understanding how epigenetic and genetic mechanisms contribute to the malignant process, we hope to identify new treatment options for incurable brain tumors.
Principal Investigator
Dr. Marco Gallo’s main interest is to understand how the epigenome defines self-renewal hierarchies in brain tumors. His ultimate goal is to identify new and effective ways to target cancer stem cells in brain malignancies.
Funding
Mapping the effects of EZHIP function with single-cell genomic approaches - #A6M05172501
Grant funding from Ependymoma Research Foundation
Targeting core malignant programs in pediatric high-grade gliomas - #1436154
Grant funding from Alex’s Lemonade Stand Foundation
Single-Cell Genomics and Epigenomics
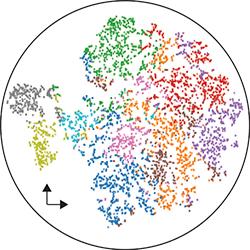
Not all cells in a brain tumor are the same. For instance, some cells are resistant to therapy, whereas others are sensitive; some divide frequently and generate lots of other tumor cells, whereas others appear dormant. Recognizing that brain tumors are ecosystems composed of many different types of cells, we adopted technologies that enable genomic profiling of individual cells, including scRNA-seq and scATAC-seq.
These platforms are leading to a better understanding of how tumor cells interact with each other and with their microenvironment, how they escape treatment and the immune system.
We are using this information to figure out ways to target tumor cells more effectively. Our wet and dry lab teams have been working together to generate single-cell -omics data from clinical specimens and our patient-derived models, perform informatics analyses to generate new hypotheses, and functionally test our predictions.
Brain Cancer 3D Genome
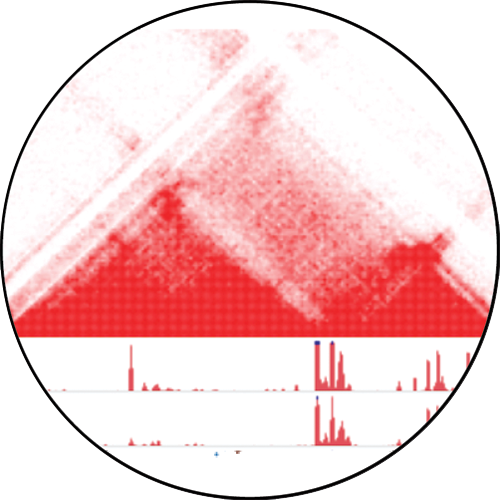
The function of a cell is determined by which genes are turned on or off. As an example, neurons and astrocytes in the brain turn on different sets of genes. The genome is organized in three-dimensional (3D) space inside the nucleus to enable some genes to be turned on while turning off other genes that are not needed in a given type of cell.
Likewise, the function and behavior of tumor cells are impacted by 3D genome architecture. Our lab has deployed new techniques – including Hi-C – to reconstruct the 3D genome of brain cancer cells.
These efforts are enabling us to understand the strategies used by the tumor to turn on genes that contribute to its aggressive behavior and to therapy resistance.
Preclinical Studies
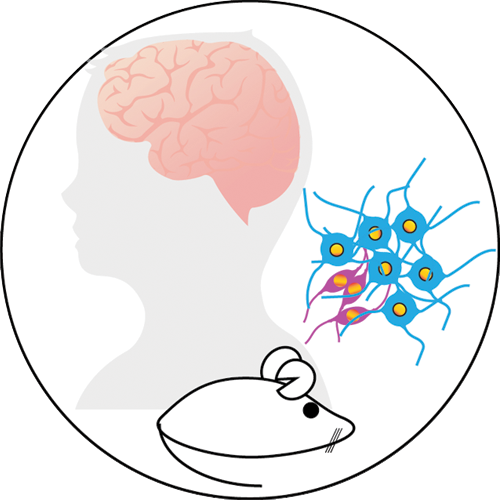
We use clinical samples – usually surgical tumor resections – to grow cell lines in the lab. These cell lines allow us to model each patient’s disease, giving us a better understanding of how brain cancers behave in different patients. They also give us an opportunity to identify biological processes that are shared across patients and that could be exploited to design new treatments.
These patient-derived models are used to understand how the genome and epigenome impact the function of malignant cells. We use molecular tools and genome engineering to turn off or on specific genes and then test their effect on cell fitness. We also transplant these patient-derived models in mice to explore the efficacy of new molecular approaches in preclinical settings.
Our preclinical models faithfully recapitulate salient features of the cancers we study and have led to close collaborations with pharmaceutical companies in attempts to translate our discoveries into better treatments for patients.









