Epigenetic Dysregulation
Disruption of chromatin regulatory systems is a widespread feature of many diseases, including cancer. One of the major chromatin regulators with frequent contributions to malignancy is the family of BAF (SWI/SNF) and PBAF complexes. These ATP-dependent chromatin remodelers harness the free energy of ATP hydrolysis to power mechanical changes of the chromatin landscape. Such changes include moving and ejecting histones from DNA, an essential activity for regulating DNA accessibility for transcription factors, topoisomerases, and state changes within the nucleus.
We and others showed that BAF and PBAF complexes are mutated in ~20% of all cancers, making them one of the most frequently mutated regulatory complexes in malignancy. In our previous work, we uncovered widespread mutational patterns in many different cancers, with high mutation frequencies analogous to other major tumor suppressors such as p53 or Rb1. In certain cancers, these mutations have been shown to drive malignancy. Unfortunately, the role of these complexes in the larger number of more frequent cancers remains mysterious. Moreover, there currently exist no targeted therapies to treat patients whose cancers have these defects.
Several of our studies focus on the BAF/PBAF ATPase subunit SMARCA4 (BRG1). SMARCA4 regulates DNA accessibility at super-enhancers, and opposes the accumulation of Polycomb repressive factors at bivalent chromatin. We are using new technologies to reveal how BAF and PBAF subunits work, and to identify new approaches to treat their dysfunction in cancer and other diseases. We are developing multi-scale approaches to assess the function of BAF and PBAF complexes from the scale of single molecules to multicellular systems. Our experiments span a variety of disease contexts, including leukemia and several solid tumors.
To read more about our research, view our publications.
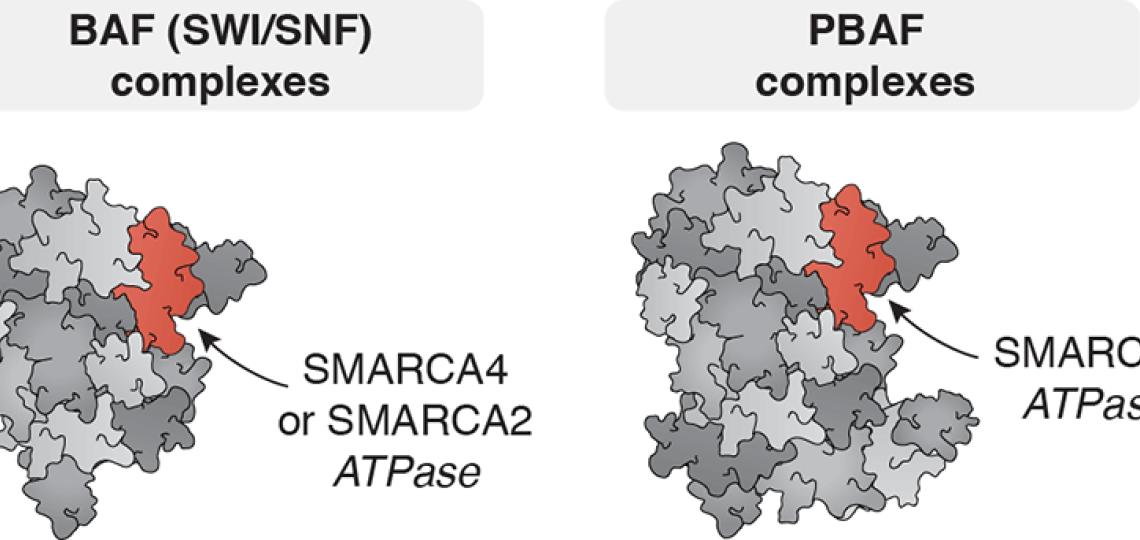
BAF (SWI/SNF) and PBAF Complexes
ATP-dependent chromatin remodelers with widespread roles in development and carcinogenesis
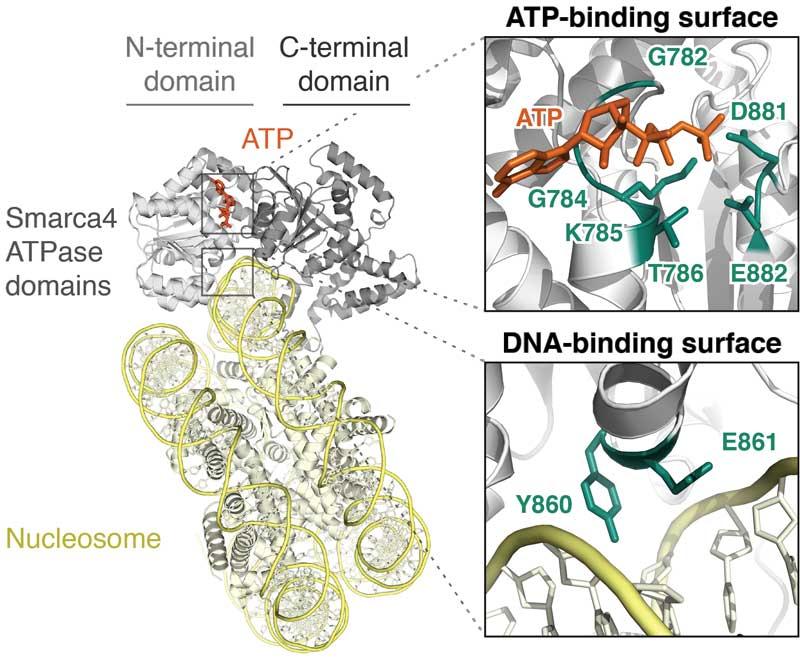
SMARCA4 (BRG1) ATPase in Malignancy
SMARCA4, the ATPase of BAF and PBAF complexes, acts as a tumor suppressor or oncogene in distinct tissues. We employ multi-scale techniques to reveal therapeutic vulnerabilities associated with this molecular motor.








