About the Lab
The Translational Pediatric Nutrition laboratory led by Dr. Douglas Burrin works on basic and translational projects designed to establish how nutritional support, enteral versus parenteral, effects gut and liver function and susceptibility to disease in early neonatal infant development. The Burrin Lab has pioneered the use of neonatal piglets to develop unique models to address clinically-relevant problems in pediatric nutrition and gastroenterology including necrotizing enterocolitis (NEC), parenteral nutrition-associated liver disease (PNALD) and neonatal obstructive cholestasis (biliary atresia).
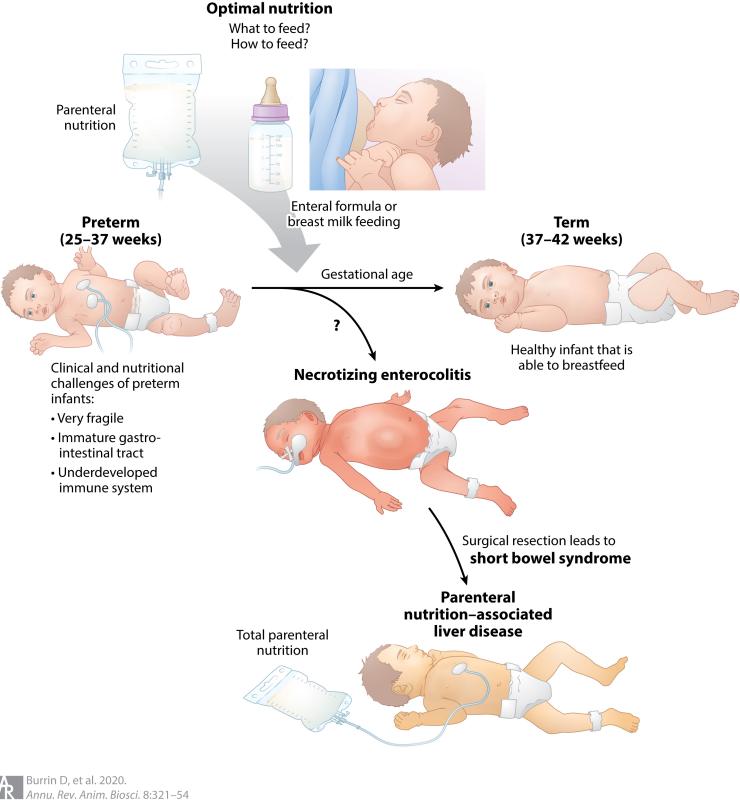
Clinical and nutritional challenges of preterm infants. Clinicians are faced with important questions about the fundamental choices of how and what to feed hospitalized preterm infants compared to term infants. These choices include intravenous parenteral nutrition (i.e., TPN) or enteral nutrition composed of breast milk or infant formula. The combination of immature intestinal digestive and immune function increases the risk for NEC in preterm infants. NEC is a devastating intestinal disease, which can lead to surgical resection of the intestine and short bowel syndrome (SBS). Prolonged parenteral nutrition resulting from GI disease (SBS) or other clinical morbidities increases the risk for PNALD.
Principal Investigator
View Dr. Douglas Burrin's bio for details on his interests and recent publications in the area of pediatric nutrition, gastroenterology and development.
Current and Past Lab Projects
- Translational Relevance of the Piglet in Pediatric Nutrition
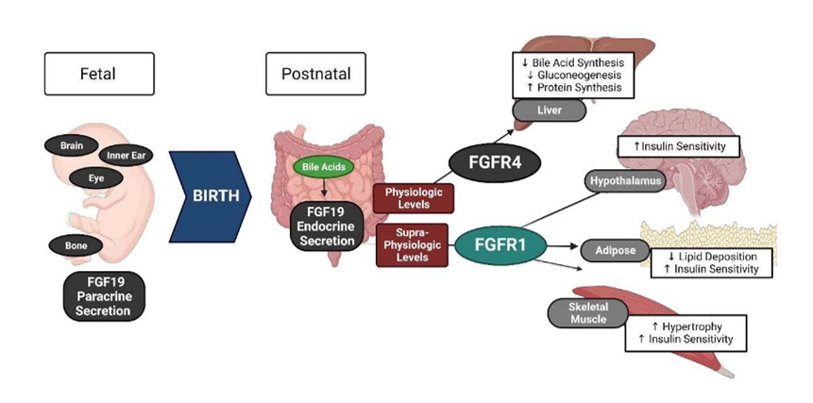
- Biology of Fibroblast Growth Factor 19 in Infancy
- Enteral Nutrition, Neonatal Intestinal Adaptation & GI Hormones
Publications
Our research projects and studies result in publications in PubMed and other scientific journals
Contact Us
Texas Medical Center
Children's Nutrition Research Center
1100 Bates Ave.
Houston, TX 77030
Appointments: (713) 798-7049
Lab: (713) 798-7093
Email: dburrin@bcm.edu
Email: doug.burrin@usda.gov
Twitter: @DougBurrin