About the Lab
In the Reimer lab, we want to understand the mechanisms underlying fast brain state changes, the downstream effects of these changes on sensory processing and behavior, and the ways that functional changes interact with and are constrained by anatomical properties (circuits and cell types).
Establishing pupillometry as an indicator of rapid brain state changes in awake mice

We were the first to show that fluctuations in pupil size track brain state changes in the mouse, opening the possibility of using the mouse as a model to study these effects, and emphasizing the importance of monitoring the pupil in order to account for variability in sensory responses. Pupil monitoring of head-fixed mice has since been widely adopted in the field.
Reimer, J., Froudarakis, E., Cadwell, C. R., Yatsenko, D., Denfield, G. H., and Tolias, A. S. (2014). Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84(2), 355–362.
Expanding our understanding of the spatial and temporal scale of neuromodulation in the cortex.
We recently performed the first simultaneous imaging of acetylcholine sensors and cholinergic axon activity in vivo, clarifying the temporal relationship between these two signals and highlighting differences in the dynamics of cholinergic release and axonal activity around locomotion and pupil dilation. With high-resolution two-photon imaging, we were able to observe that acetylcholine levels fall off with distance from cholinergic axons and that rapid clearance occurs for small transients, providing insights into the precise spatiotemporal characteristics of cortical acetylcholine signaling in vivo. A complementary manuscript is in preparation on norepinephrine dynamics.
Neyhart, E., Zhou, N., Munn, B. R., Law, R. G., Smith, C., Mridha, Z. H., Blanco, F. A., Li, G., Li, Y., Hu, M., McGinley, M. J., Shine, J. M., & Reimer, J. (2024). Cortical acetylcholine dynamics are predicted by cholinergic axon activity and behavior state. Cell Reports, 43(10). https://doi.org/10.1016/j.celrep.2024.114808. PMID: 39383037.
Dataset: https://dandiarchive.org/dandiset/001176/draft
Funding: R01 NS128901-01 The Spatial and Temporal Scale of Neuromodulation in Mouse Sensory Cortex
Studying changes in neuromodulation across the lifespan and in Alzheimer’s disease.
We are using high-resolution fluorescence imaging in transgenic mice to investigate changes in the dynamics of four key neuromodulators—acetylcholine, norepinephrine, dopamine, and serotonin—during normal aging and Alzheimer's disease progression. The goal of this project is to create a multimodal atlas that links characteristic changes in fast neuromodulator dynamics with anatomical and behavioral markers of aging and disease. A key objective is to explore the translational potential of pupillometry as a non-invasive diagnostic tool for early detection of neuromodulatory dysfunction in Alzheimer's disease. This work is part of a collaborative project with Jeannie Chin (BCM), and Read Montague and Matthew Howe (Virginia Tech).
Neyhart, E., Zhou, N., Chin, J., & Reimer, J. (2024). Arousal-linked levels of norepinephrine and acetylcholine in the cortex change across the lifespan in a mouse model of Alzheimer’s Disease [Poster Presentation]. Neuroscience 2024, Society for Neuroscience, Chicago, IL.
Funding: R56 AG080735 - 01A1 The Dynamic Neuromodulome in Alzheimer's Disease and Aging
Comparing changes in neuromodulators during alertness and attention across species
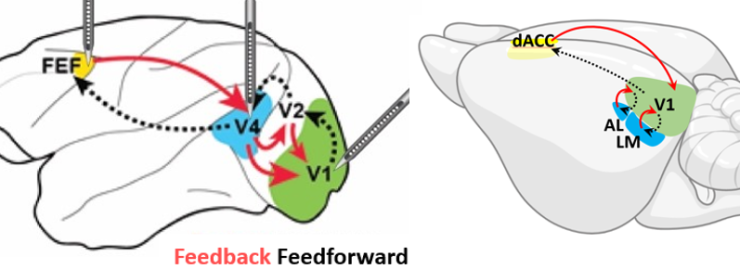
This project develops the first systematic framework to directly compare the neural mechanisms of global arousal and selective attention across mice and monkeys, bridging a critical gap between traditionally separate research fields. Using innovative cross-species behavioral tasks, advanced neurotransmitter sensors, and optogenetic techniques, we aim to determine whether attention and arousal operate through shared or distinct neural pathways. (This work is a collaboration with Valentin Dragoi at Methodist Hospital)
Funding: R34 NS137454 - 01 Cross-Species comparison of cholinergic neuromodulation in mice and primates
Determining the role of astrocyte calcium on cortical circuits
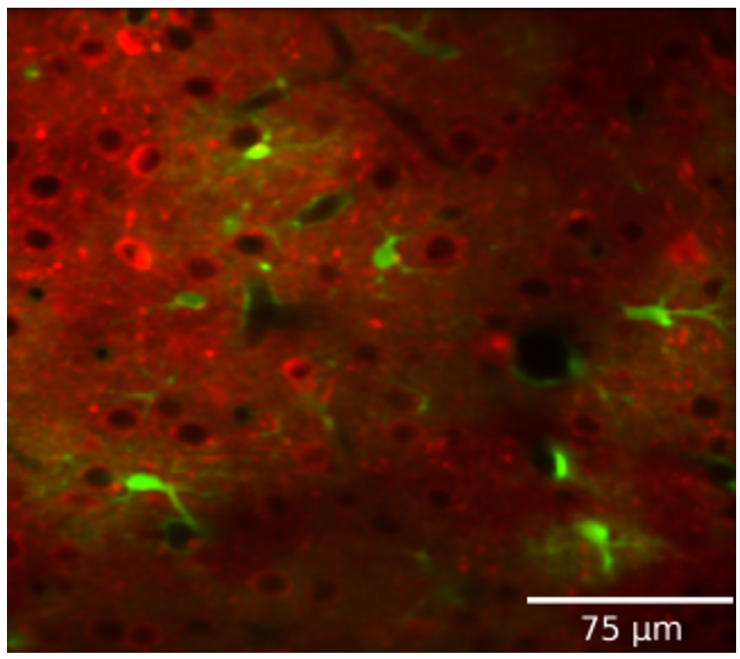
Astrocytes integrate local changes in neural activity and global neuromodulatory signals, and respond with intracellular calcium increases. We have developed a pipeline to image and analyze astrocytic calcium events in vivo, along with neural calcium activity, neuromodulators (ACh and NE), or glutamate sensors. We can also test the role of astrocyte calcium events on local neural activity by reducing astrocyte calcium with either local expression of CalEx (a constitutively-active calcium pump), or knockout of IP3 receptor subtypes. We have expanded this approach in collaboration with Hyun Kyoung-Lee (BCM) to look at the effects of Slc4a4 knockout in astrocytes. In collaboration with Fabien Sinz (guttenberg) we are also exploring computational (machine learning) methods that may be able to capture a higher resolution picture of the temporal and spatial coordination of astrocyte calcium with local neurons.
Developing and applying new tools for extracting scientific insights from large-scale electron microscopy connectomes
We developed a computational pipeline called NEURD, which decomposes and annotates neural reconstructions from EM, in order to enable downstream analyses and automated proofreading. We used this pipeline to perform morphological cell-typing, spine analyses, and connectomics analyses that generated multiple novel findings about the microscale architecture of neurons in the MICrONS cortical volume. We are currently extending NEURD to support large-scale automated proofreading across multiple millimeter-scale EM reconstructions.
Celii, B., Papadopoulos, S., Ding, Z., Fahey, P. G., Wang, E., Papadopoulos, C., Kunin, A. B., Patel, S., Bae, J. A., Bodor, A. L., Brittain, D., Buchanan, J., Bumbarger, D. J., Castro, M. A., Cobos, E., Dorkenwald, S., Elabbady, L., Halageri, A., Jia, Z., … Reimer, J. (2025). NEURD offers automated proofreading and feature extraction for connectomics. Nature, 640, 487–496. https://doi.org/10.1038/s41586-025-08660-5
GitHub: https://github.com/reimerlab/NEURD.git
Funding: RF1 MH130416-01 Anatomical connectivity and activity in primary visual cortex of mouse
U01 NS137250 - 01 BRAIN CONNECTS: A Scalable Automated Proofreading Framework for Connectomics
Studying the relationship between function and structure in the visual system
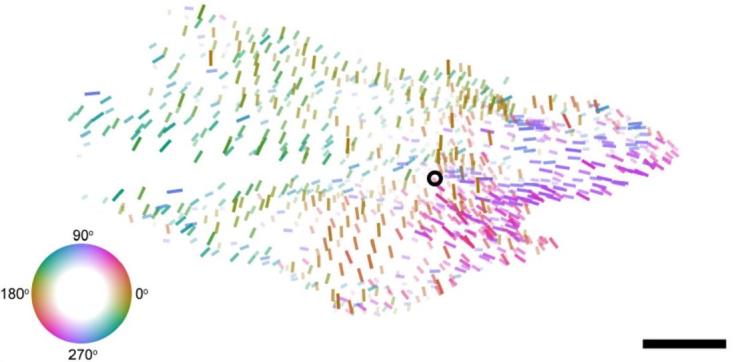
Our collaboration on the MICrONS project helped reveal a fundamental "general wiring rule" in mouse visual cortex, demonstrating that excitatory neurons with similar response properties are more likely to be synaptically connected at scales spanning multiple visual areas. In a separate but related series of experiments, we have described a smoothly-varying global map of orientation preferences spanning multiple areas of mouse visual cortex—challenging the long-held "salt-and-pepper" model of orientation tuning in the mouse. (collaboration with Andreas Tolias Lab)
Ding, Z., Fahey, P. G., Papadopoulos, S., Wang, E. Y., Celii, B., Papadopoulos, C., Chang, A., Kunin, A. B., Tran, D., Fu, J., Ding, Z., Patel, S., Ntanavara, L., Froebe, R., Ponder, K., Muhammad, T., Bae, J. A., Bodor, A. L., Brittain, D., … Tolias, A. S. (2025). Functional connectomics reveals general wiring rule in mouse visual cortex. Nature, 640, 459–469. https://doi.org/10.1038/s41586-025-08840-3
Fahey, P. G., Muhammad, T., Smith, C., Froudarakis, E., Cobos, E., Fu, J., Walker, E. Y., Yatsenko, D., Sinz, F. H., Reimer, J., & Tolias, A. S. (2019). A global map of orientation tuning in mouse visual cortex. bioRxiv. https://doi.org/10.1101/745323
Datasets: Microns explorer: https://www.microns-explorer.org/
GitHub: https://github.com/cajal/microns-funconn-2025
Funding: RF1 MH130416-01 Anatomical connectivity and activity in primary visual cortex of mouse
U01 NS137250 - 01 BRAIN CONNECTS: A Scalable Automated Proofreading Framework for Connectomics
Examining the representation of odors in the olfactory bulb using wide-field two-photon imaging
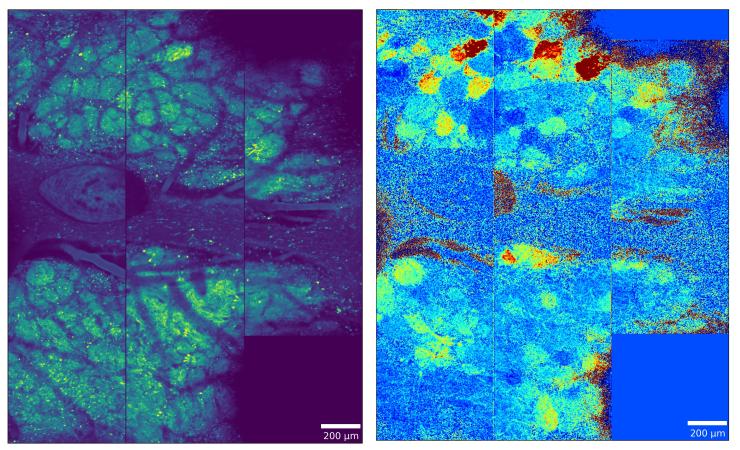
Using wide-field two-photon calcium imaging to record from large populations of glomeruli across the dorsal olfactory bulb, this research challenges traditional sparse coding models by demonstrating that odor information is redundantly encoded in dense, persistent neural representations that can accurately classify odors even when highly active glomeruli are excluded. Complementary comparative work reveals conserved computational principles across mammalian and insect olfactory systems, including anticorrelated ON and OFF responses that enhance odor contrast and create odor-specific "afterimages" that persist long after stimulus termination. Together, these studies reveal fundamental organizing principles of olfactory processing that prioritize robust, redundant encoding over sparse representation, providing new insights into how chemical information is reliably transformed into behaviorally relevant neural codes. This work is part of a multi-lab collaboration.
Pirhayati, D., Smith, C. L., Kroeger, R., Navlakha, S., Pfaffinger, P., Reimer, J., Arenkiel, B. R., Patel, A., & Moss, E. H. (2024). Dense and Persistent Odor Representations in the Olfactory Bulb of Awake Mice. Journal of Neuroscience, 44(39). https://doi.org/10.1523/JNEUROSCI.0116-24.2024
Ling, Doris, Elizabeth H. Moss, Cameron L. Smith, Ryan Kroeger, Jacob Reimer, Baranidharan Raman, and Benjamin R. Arenkiel. "Conserved neural dynamics and computations across species in olfaction." bioRxiv (2023).
Dataset: https://dandiarchive.org/dandiset/001170/draft
Funding: UF1 NS111692-01 Multilevel Analysis of Neuronal Computations Underlying the Robust Encoding of Sensory Information in the Mammalian Olfactory System
ASAP 025194 Olfactory Circuits: a-Synuclein-Rich Neurons Respond to Environmental Triggers at the Origin of Parkinson Disease
Benchmarking and validating novel voltage sensors with Francois St-Pierre in vivo
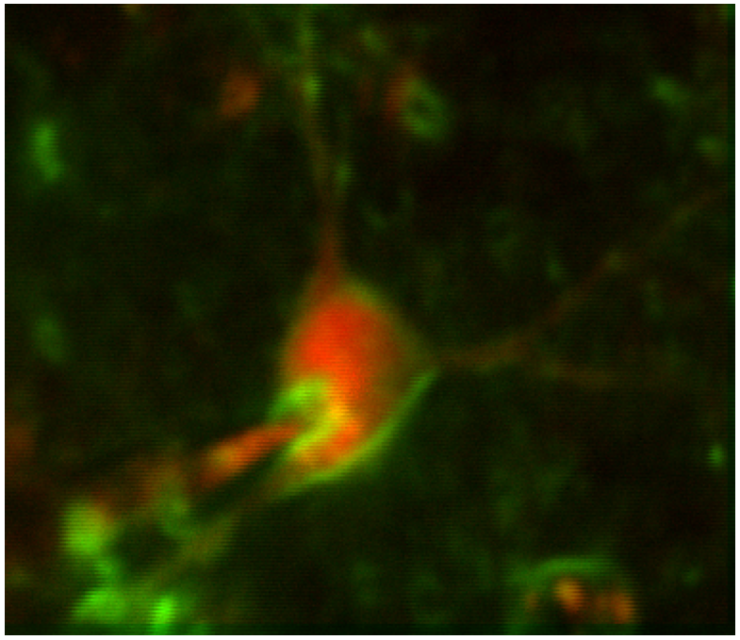
Taking advantage of our expertise with two-photon imaging and in vivo whole-cell patching, we continue to perform in vivo validation experiments for new voltage sensors from the St-Pierre lab under a variety of different contexts.
Liu, Z., Lu, X., Villette, V., Gou, Y., Colbert, K. L., Lai, S., Guan, S., Land, M. A., Lee, J., Assefa, T., Zollinger, D. R., Korympidou, M. M., Vlasits, A. L., Pang, M. M., Su, S., Cai, C., Froudarakis, E., Zhou, N., Patel, S. S., … St-Pierre, F. (2022). Sustained deep-tissue voltage recording using a fast indicator evolved for two-photon microscopy. Cell, 185(18), 3408-3425.e29. https://doi.org/10.1016/j.cell.2022.07.013
Funding: R01 NS136027-02 Red-shifted voltage indicators for two-photon all-optical physiology and multi-spectral imaging
R01 NS146078-01 Advanced voltage indicators tailored for two-photon resonant scanning applications
Voltage imaging: dendrites
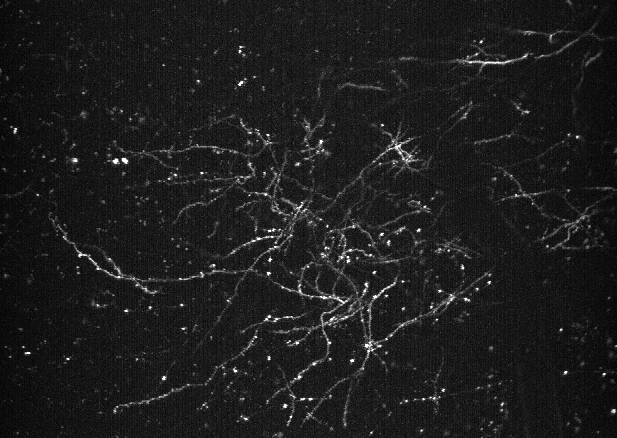
To date, techniques for monitoring dendrite voltage in vivo have mostly been limited to difficult dendritic patch experiments in proximal dendrites. However, novel GEVIs have the potential to enable observation of membrane potentials in distal dendrites at high spatial and temporal resolution. We have demonstrated that these sensors can be utilized in dendrites to recover subtle changes in membrane voltage related to brain state.
Land, M. A., Galdamez*, M. A., Villette*, V., Zhu*, J., Lu*, X., Marosi*, M., Yang, S., McDonald, A., Dong, X., Zaabout, E., Liu, H., Liu, Z., Colbert, K. L., Lai, S., Shorey, M., Ayon, A., Bradley, J., Mailhes-Hamon, R. G., Natan, J., … Reimer, J., St-Pierre, F. Designer indicators for two-photon recording of subthreshold voltage dynamics. In press at Nature Communications*These authors contributed equally
Funding: R34 NS132045-02 Validation and Optimization of Two-Photon Dendritic Voltage Imaging in Vivo
Voltage imaging: Astrocytes
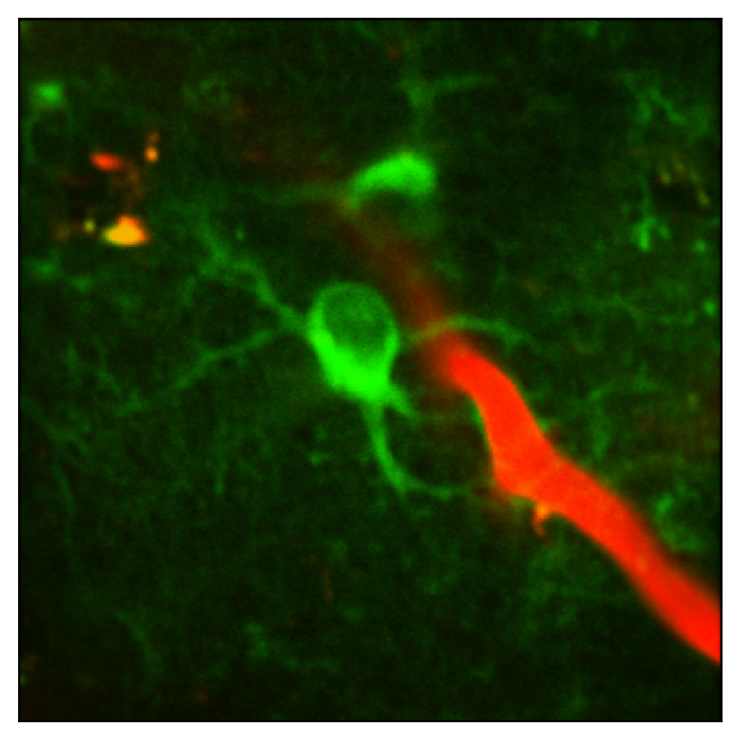
The goal of this project is to investigate the role of astrocyte voltage in state-dependent sensory processing, in vivo. In a manuscript currently in preparation for submission, we demonstrate that standard two-photon imaging of novel GEVIs enables reliable measurement of astrocyte voltage dynamics in vivo in behaving mice. Our preliminary data indicate that robust depolarizations in astrocyte membrane voltage (Vm) are correlated with intense periods of local neural activity and periods of increased cholinergic and noradrenergic tone. We hypothesize that this coordination of astrocyte voltage with neural activity may enable astrocytes to use Vm changes as an informative signal to adjust their homeostatic functions.
Paper on Biorxiv soon!
Funding: R01 NS146023-01 In Vivo Imaging of State-Dependent Astrocyte Voltage Dynamics and Neural Activity in Mouse Visual Cortex
S10 microscope
The Reimer lab led a successful proposal to bring a $1M+ Femtonics microscope to the BCM Neuroscience department. This microscope is ideal for fast scanning of voltage indicators in spatially-distributed targets.
Funding: S10 OD038398-01 Three-dimensional random access acousto-optical scanning two-photon microscope








