Ongoing Projects
- Posttranslational modification of transcription factors controlling oocyte growth and differentiation
- Role of SUMOylation in mammalian meiosis
- Function of the BMP family in ovary development
- Mouse models of female infertility
- Development of novel non-invasive diagnostics for early ovarian cancer detection
Previous Projects
Novel Non-Invasive Detection of Ovarian Cancer
Ovarian cancer is the fifth most common cancer in women and is responsible for the most gynecological disease-related deaths. The majority of cases are not detected until late stage disease when prognosis is poor, partly due to vague and subtle symptoms such as bloating, constipation, feeling full quickly, abdominal and pelvic pain. Current biomarkers used clinically have been largely ineffective at detecting early stage ovarian cancer. Therefore, the discovery of novel early detection methods are greatly needed. Our lab is currently exploring different methodologies to detect ovarian cancer biomarkers in multiple biofluids and tissues. We hope that such techniques will not only reduce the morbidity and mortality of this disease, but also decrease stress and concern for women undergoing evaluation for common symptoms of ovarian cancer.
TGFbeta Signaling in Juvenile Granulosa Cell Tumors of the Ovary
Juvenile granulosa cell tumors of the ovary are sex cord-stromal tumors that are poorly understood and treatment regimes are not well defined. The majority of juvenile granulosa cell tumors affect girls younger than 20 years of age. Our lab utilizes mouse models of the disease, as well as human cell lines, to investigate aberrations in the transforming growth factor beta (TGFbeta) signaling pathway that drive the development and growth of these tumors. By understanding the mechanisms of tumorigenesis, we can develop better targeted therapies for the treatment of this cancer.
Determine the Expression Pattern of BMP Signaling in the Developing and Adult Reproductive Tract
Bone morphogenetic protein signaling is an essential pathway for the development of many organisms. BMPs have also been identified to regulate fertility. However, the exact patterning of BMP signaling in the developing reproductive tract is unknown. We are using a GFP promoter mouse to monitor BMP activity in the developing reproductive tract. By knowing which cells respond to BMPs, we will be better positioned to determine the normal versus pathologic roles of BMPs in our other mouse mutants that have defective BMP signaling.
Also, we will be able to design specific interventions for particular cell targets as novel therapeutics or to assist with artificial reproductive technologies.
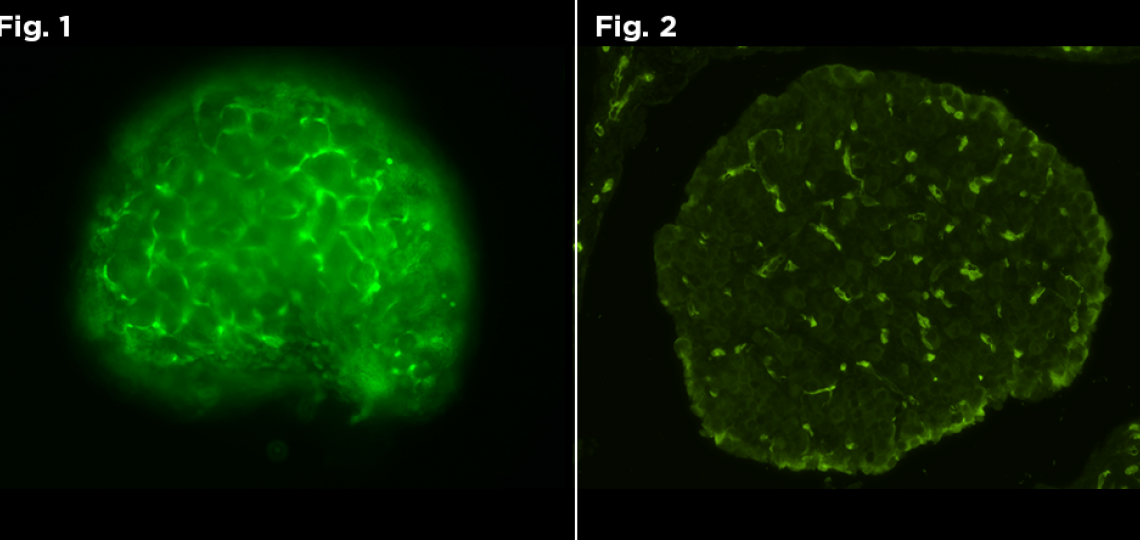
Figure 1: Live Fluorescent image of GFP positive neonate. Figure 2: Fluorescent image of GFP positive neonate.
Protein SUMOylation is Essential for Oocyte Development and Female Fertility
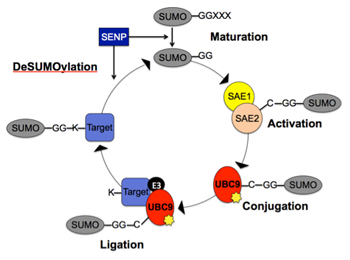
Early menopause or premature ovarian failure has negative health repercussions, including increased heart disease, bone loss, sexual dysfunction or even early death. The age at menopause is largely determined by the number of oocytes found in the non-growing pool of oocytes (the "ovarian reserve"), which appears to set near birth in both mouse and human ovaries. Because POF is both idiopathic and generally nonsyndromic, mouse models have been instrumental in identifying POF candidate genes, which include oocyte-specific transcription factors. Our research interests involve investigating how post-translational modifications, specifically SUMOylation, of these transcription factors regulates ovarian folliculogenesis and oocyte development. Understanding these underlying regulatory mechanisms may hold the key to managing fertility in women with POF, or to uncovering the basis of their disease.
Early menopause or premature ovarian failure has negative health repercussions, including increased heart disease, bone loss, sexual dysfunction or even early death. The age at menopause is largely determined by the number of oocytes found in the non-growing pool of oocytes (the "ovarian reserve"), which appears to set near birth in both mouse and human ovaries. Because POF is both idiopathic and generally nonsyndromic, mouse models have been instrumental in identifying POF candidate genes, which include oocyte-specific transcription factors. My research interests involve investigating how post-translational modifications, specifically SUMOylation, of these transcription factors regulates ovarian folliculogenesis and oocyte development. Understanding these underlying regulatory mechanisms may hold the key to managing fertility in women with POF, or to uncovering the basis of their disease.








