Click a button to explore delayed gamma activation
Delayed Gamma Theory
|
The nucleus of an atom contains protons and neutrons. Protons carry a positive charge, opposite to that of the negatively charged electrons surrounding the nucleus. The mass of protons and neutrons is similar, and each is about 1800 times larger than an electron. A neutral atom contains equal numbers of protons and electrons. An element is distinguished by the number of protons in its nucleus. Changing the number of protons would change the element's identity. However, elements can have different numbers of neutrons. These varieties are called isotopes or nuclides of an element. Chemical characteristics are not altered by changes in the number of neutrons, thus all isotopes have the same chemical behavior.
|
|
One of the elements measured by delayed gamma neutron activation is calcium. The most abundant nuclide of calcium is 40Ca, containing 20 protons and 20 neutrons. The conventional form for displaying this element is shown here. The mass number (protons + neutrons) of 40 is listed along with the chemical symbol for calcium (Ca). Almost 97% of all calcium exists in the form of 40Ca.
|
most abundant form of calcium |
|
Some nuclides are unstable. When a nuclide is unstable, it may emit certain particles, such as neutrons, electrons, or gamma rays. (see the radiation section for more details.) The particles produced and their energies are characteristic of that nuclide. Gammas emitted can be measured by detectors in some of the Bodycomp Lab counters. In delayed gamma neutron activation, incoming neutrons interact with certain elements, such as calcium. A stable atom receives a neutron and is transformed into a different nuclide with a measureable decay time (an isomer). The nuclide of calcium that behaves this way is 48Ca. 48Ca is stable, but only about 0.2% if calcium exists in this form.
|
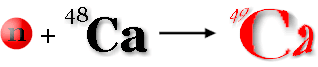
|
An incoming neutron changes stable 48Ca to unstable 49Ca, which has a half life of less than 9 minutes.
|
| As 49Ca decays, it emits certain particles at certain energies. The 3.1 MeV Gamma rays produced are detected and counted by the whole body counter. The resulting number of counts is converted to grams, representing the total amount of calcium in the whole body. |
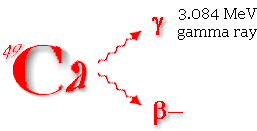 |
USDA/ARS
Children's Nutrition Research Center
1100 Bates Street, Houston, Texas 77030
© Copyright 2000-2007 Baylor
College of Medicine. All Rights Reserved.
Contact
Us.