Can we Regenerate Sensory Hair Cells of the Inner Ear?
Hearing loss is one of the most common disabilities in the United States, with approximately half of all adults suffering from some degree of hearing loss by the time they reach retirement age. Hearing loss can be due to the effects of aging, or to specific environmental insults such as industrial or recreational noise or exposure to aminoglycoside antibiotics and platinum-based chemotherapy drugs. Noise-induced hearing loss has also been a huge problem in combat veterans returning from Iraq and Afghanistan.
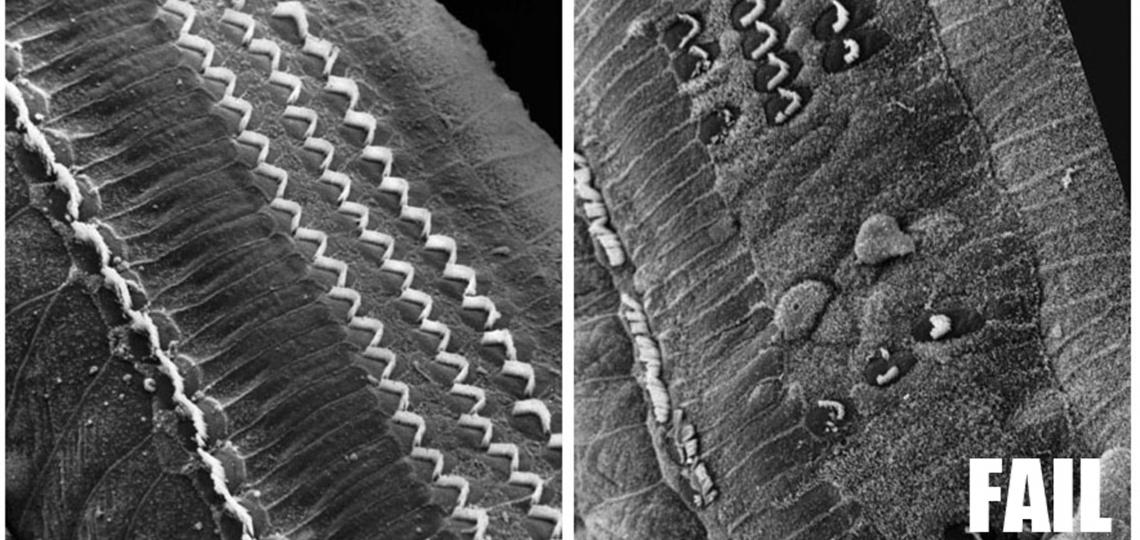
The most common form of hearing loss is caused by the death of cochlear hair cells in the organ of Corti, and once lost, hair cells in humans and other mammals do not regenerate. In contrast, non-mammalian vertebrates can functionally recover from deafening injury by mobilizing supporting cells in the cochlea to divide and differentiate to replace lost hair cells. Since the discovery of hair cell regeneration in birds in the 1980s, research has focused on trying to understand the cellular and molecular mechanisms underlying regeneration and why these processes do not occur in mammals.
In the past 10 years, a number of labs including ours have shown that mammalian supporting cells from new born mice have a limited and transient ability to re-enter the cell cycle and produce hair cells. However, by the time mice are able to hear, at two weeks of age, this capacity for proliferation has been largely lost. Since no new supporting cells are formed after birth, this suggests that the maturation of supporting cells must in some way lead to a loss of their regenerative potential.
The Notch signaling pathway is activated as hair cells and supporting cells differentiate, and has been recently shown to be necessary to maintain the differentiated state of hair cells and supporting cells in early postnatal life. Notch signaling is also activated during hair cell regeneration in birds, and we and others have shown that disruption of Notch signaling in the mammalian cochlea causes supporting cells to trans-differentiate into hair cells. Once again, the ability of supporting cells to do this declines with age – they show a robust response to a blockade of the Notch signaling pathway at the time of birth, but a complete lack of response to such blockade just a few days later. We recently used RNA-seq to identify transcriptional changes in supporting cells in the first postnatal week, and we compared these transcriptomes to those of supporting cells cultured in the presence of Notch pathway inhibitors. Strikingly, we found that the transcriptional response to Notch blockade disappears almost completely in the first postnatal week.
Finally, attention has focused on several pathways and molecules implicated in hair cell development and regeneration. The basic helix-loop-helix transcription factor Atoh1 is one of the first transcription factors to be switched on in hair cells as they differentiate. Atoh1 has been demonstrated to be both necessary and sufficient for the development of hair cells, but its ability to induce hair cells declines rapidly after birth. We have collaborated with Huda Zoghbi’s lab at Baylor to identify direct targets of Atoh1 in cochlear and vestibular hair cells, and we are now testing whether epigenetic silencing contributes to the loss of regenerative ability in the mammalian cochlea.
Maass, J.C., Gu, R., Basch, M.L., Waldhaus, J., Martin-Lopez, E., Xia, A., Oghalai, J.S., Heller, S. and Groves, A.K. (2015). Changes in the regulation of the Notch signaling pathway are temporally correlated with regenerative failure in the mouse cochlea. Frontiers in Cellular Neuroscience 9, 110
Cai, T., Jen, H.-I, Kang, H., Klisch, T.J., Zoghbi, H.Y and Groves, A.K. (2015). Characterization of the transcriptome of nascent hair cells and identification of direct targets of the Atoh1 transcription factor. Journal of Neuroscience 35, 5640-5654 (Supplementary data for Cai et al. (2015) can be downloaded here. Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table Legends)
Cai, T, Seymour, M.L., Zhang, H., Pereira, F. A. and Groves, A.K. (2013). Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. J. Neuroscience 33, 10110-10122.
Groves, A.K., Zhang, K.D., and Fekete, D.M. (2013). The genetics of hair cell development and regeneration. Ann. Rev. Neuroscience, 36, 361-381.
White, P.M., Stone, J.S., Groves, A.K. and Segil, N. (2012). EGFR signaling is required for regenerative proliferation in the cochlea: Conservation in birds and mammals. Developmental Biology 363, 191-200.
Groves, A.K. (2010). The challenge of hair cell regeneration. Experimental Biology and Medicine, 235, 434-446.
Doetzlhofer, A., White, P.M., Lee, Y.-S., Groves, A.K. and Segil, N. (2006). Prospective identification and purification of hair cell and supporting cell progenitors from the embryonic cochlea. Brain Research 1091, 282-8.
White, P.M., Doetzlhofer, A., Lee, Y.-S., Groves, A.K. and Segil, N. (2006). Cochlear supporting cells retain the ability to divide and transdifferentiate into sensory hair cells. Nature 441, 984-987.







