HTLV-1 Associated Myelopathy
This 51 year old man presented with three years of progressive bilateral leg stiffness, weakness, and worsening gait, accompanied by one year of mild right hand weakness and symptoms of intermittent distal leg numbness. He occasionally was incontinent of urine and stool. Furthermore, he complained of episodic horizontal diplopia. Of particular importance, his medical history was remarkable for a blood transfusion 15 years ago, and a diagnosis of lower extremity axonal neuropathy during the previous year, without a documented cause. His neurological examination was notable for significant atrophy in the leg muscles, findings suggestive of myelopathy, and a VI nerve palsy. MR imaging of the brain and spinal cord did not demonstrate an anatomic cause of these findings. Electromyography and nerve conduction studies were consistent with the previous diagnosis of axonal neuropathy. CSF examination was abnormal, with mildly elevated protein, and three oligoclonal bands detected by high-resolution electrophoresis.
Several diagnoses were considered as potential explanations of this patient's findings. Multiple sclerosis could produce multifocal disease of the CNS, with discrete attacks, and may be accompanied by a peripheral neuropathy in about 5% of cases. However, the associated neuropathy is typically demyelinating in nature, and the diagnosis of MS rests on exclusion of alternative causes. Vasculitic syndromes, such as polyarteritis nodosa, may produce both CNS lesions and a peripheral neuropathy, typically either multifocal (mononeuropathy multiplex) or diffuse axonal in nature. However, most of these syndromes follow a more aggressive course than in this patient, and are typically accompanied by abnormal screens for systemic inflammation. Other, more indolent, systemic inflammatory disorders, such as Sjogren's syndrome, sarcoidosis, and systemic lupus erythematosus, could produce CNS and peripheral neuropathic involvement. In this patient, however, the absence of suggestive clinical symptoms, and negative screening studies, lowered our clinical suspicion for these diagnoses. Vitamin B12 deficiency can produce myelopathy and neuropathy, and should always be screened in patients with this type of presentation. Rarely, adult-onset adrenoleukodystrophy, presenting as adrenomyeloneuropathy, can be identified as a cause of myelopathy and neuropathy. The late age of onset, normal electrolytes, and lack of characteristic MRI findings argues against this unlikely possibility. Paraneoplastic syndromes may occasionally present with myelopathy and neuropathy, but the slow clinical course and negative screening studies did not raise high concern for malignancy in this patient. Infectious diseases, notably the retroviruses, may also produce multifocal disease of the central and peripheral nervous system. This patient did not test positive for HIV, and showed no evidence of lymphadenopathy or immunosuppression. However, a screening test for antibodies to HTLV-1 was positive, and a diagnosis of HTLV-1 associated myelopathy (HAM) in this patient was supported by confirmatory PCR and by demonstrating antibodies to HTLV-1 in the patient's CSF.
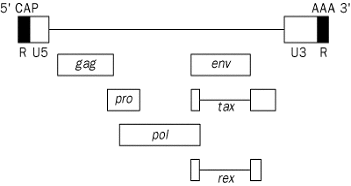
Figure 1. Genomic structure of HTLV-1
HTLV-I is an enveloped, double-stranded RNA, type C virus, of the Retroviridae family. This virus is trophic for CD4+ T-cells, and stimulates T-cell proliferation. The HTLV-1 RNA encodes structural genes: gag (encodes p19 and p24) and env (encodes gp21 and gp46). Viral enzymes include the reverse transcriptase (pol), integrase and protease (pro), the latter acting to process gag proteins. There are two regulatory proteins: a transcriptional transactivator (tax), and an mRNA splicing and transport regulator (rex). Two long terminal repeat sequences (U5, U3) control proviral transcription (see Figure 1; Manns et al., 1999 Lancet).
Adult T-cell lymphocytic leukemia develops in 2-3% of HTLV-1 seropositive patients. HTLV-1 associated myelopathy (HAM), also known in the Gulf states as tropical spastic paraparesis (TSP) develops in 1-2% of HTLV-1 seropositive patients. Fewer than 2% of HTLV-1 seropositive patients develop other inflammatory conditions, including uveitis, dermatitis, polymyositis, HTLV-1 associated arthropathy and Sjogren's syndrome. About ninety-five percent of HTLV-1 seropositive patients appear to be asymptomatic carriers.
Endemic areas for HTLV-1 occurrence include the Caribbean, 3-4% seropositive, north to equatorial Africa, and southwest Japan. Nine percent of IV drug users in the US are seropositive, and 0.025% of 39,898 blood donors in a US study were seropositive. The female seropositive prevalence is up to twice that of males (Edlich et al., 2000 J Emer Med; McKendall RR, ed. 1989 Handbook of clinical neurology). Transfusion is the most efficient method of HTLV-I transmission, with a probability of seroconversion of 40-60% following exposure to infected blood products. The median time to seroconversion is 51 days following exposure. The virus is transferred by the cellular components of blood, including packed red cells, whole blood, and platelet fractions; fresh-frozen plasma is believed to be low risk. Vertical transmission may occur by ingestion of the mother's milk, at a rate of 18-30%. Transmission by sexual contact occurs at rates of approximately 4.9% in females married to infected males and approximately 1.2% in males married to infected females (Manns et al., 1999 Lancet).
Symptoms typically become evident during the 4th and 5th decades of life, and are usually dominated by a slowly progressive spastic paraparesis. Ninety-seven percent of affected patients have spasticity with increased tendon reflexes, clonus and Babinski signs in the lower extremities. The vast majority (94%) also have urinary bladder dysfunction; 88% have lower-extremity weakness; 65% have sensory disturbances; 5% have cerebellar ataxia (Osame et al., 1990 Hematol Rev). A number of patients also have bulbar and/or subcortical findings that may mimic disorders such as multiple sclerosis. Occasionally, peripheral neuropathy is seen in association with HTLV-1 infection. These observations make it clear that HTLV-1 associated disease is not restricted to involvement of the spinal cord, as is often implied in classical descriptions. Diagnosis of tropical spastic paraparesis by clinical criteria typically involves documenting the following:
With the advent of more reliable tests for HTLV-1, it is becoming clear that HTLV-1 associated neurologic disease can present in ways that fall outside the traditional criteria for clinical diagnosis of TSP. For instance, sensory levels may be appreciated, suggesting transverse involvement of the cord rather than disease of isolated tracts. The cranial nerves may be involved, including (uncommonly) the optic nerve. Finally, apparent exacerbations of illness may occur.
In the first two months of HTLV-1 infection, antibodies to gag are dominant (anti-p24 appears before anti-p19). Antibody responses to gp21 appear next, then antibodies to gp46 and tax. As previously noted, a latent period may occur in which HTLV-1 viremia is present, without detectable antibody responses (Manns et al., 1999 Lancet).
Twenty-five to sixty percent of patients with HTLV-1 infection and neurologic findings have a mild CSF pleocytosis, and slightly less than 50% of patients have a mildly elevated CSF protein. Most patients show oligoclonal bands on CSF electrophoresis, associated with intrathecal synthesis of antibodies to HTLV-1. There is marked elevation of neopterin in CSF of patients with HTLV-1 associated myelopathy (neopterin is released by macrophages or microglia when stimulated by T-lymphocytes, and is felt to be a marker for activation of cellular immunity). Also, unusually shaped, "flower-like" lymphocytes are seen in the serum and CSF of patients infected with HTLV-1 (Edlich et al., 2000 J Emer Med). Confirmation of positive serologic studies for HTLV-1 may be accomplished by PCR amplification of proviral DNA fragments from infected lymphocytes. Demonstration of intrathecal antibodies to HTLV-1 is felt to support a diagnosis of HTLV-1 associated myelopathy.
Increased signal intensity in the brain white matter can be seen on T2-weighted MR images, and periventricular white matter may also show demyelination in a pattern similar to that of multiple sclerosis. MRI of the spinal cord may show changes suggestive of active or chronic demyelination; occasionally, swelling or atrophy of the spinal cord may occur, particularly in the thoracic region.
Electrodiagnostic studies may be helpful in the diagnosis of HTLV-1 associated myelopathy. The most common finding on evoked potential studies is delayed lower limb somatosensory evoked potentials. Visual and brainstem auditory evoked potentials are occasionally abnormal, supporting the contention that HTLV-1 associated disease may extend beyond the spinal cord. Some patients have a demyelinating peripheral neuropathy, whereas other patients have an axonal peripheral neuropathy. Electromyographic studies showing evidence of active denervation (fibrillations and positive sharp waves), especially in the lower thoracic paraspinal muscles, may raise the suspicion of HTLV-1 associated disease (Edlich et al., 2000 J Emer Med).
There is no definitive therapy for this disorder. Therapeutic trials in about 200 patients with HAM/TSP showed varying results (Nakagawa et al., 1996 J Neurovirol). Oral prednisolone improved motor disability scores more than one grade in 69% (91/131) of patients. Plasmapheresis and lymphocytapheresis improved 44% (7/16) of patients more than one motor grade. Intrathecal hydrocortisone, intravenous high-dose methylprednisolone, and interferon-a improved smaller percentages (20-40%) more than one grade. Another study by Yamasaki et al. (1997 J Neurol Sci) showed that high dose interferon-a reduced HTLV-1 proviral DNA and improved motor performance in 5/7 patients with HAM; one patient in this series worsened, also exhibiting an increase in measured proviral DNA. CD4+ T cell autoproliferation was also markedly depressed in the responders. CD8+DR+ T cells and soluble IL-2 receptor levels in sera increased in all patients. These studies, while uncontrolled and generally of small numbers, suggest that decreasing measured HTLV-1 proviral DNA levels and T-cell immunomodulation by long term interferon-a may have a role in producing clinical benefit. However, further studies are needed to verify these promising reports.
We thank Paul Schulz, M.D., of the Houston VA Neurology Service, for contributing to the development of this case.
-- Dennis R. Mosier, M.D., Ph.D.
Email comments: