Herpes Simplex Encephalitis (HSV Type 1)
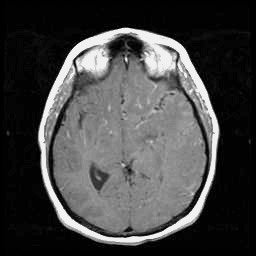
T1 post-contrast
This case illustrates many of the diagnostic challenges that may be encountered in patients presenting with herpes simplex infection of the brain. The patient had presented with transient focal sensory symptoms, followed by a witnessed seizure, and only on the third day of her illness began to exhibit fever and progressive decline in mental status. At this point, treatment was empirically initiated with antibacterials and with acyclovir, although EEG studies and a high quality MRI study were unrevealing. Two successive CSF examinations, on days 2 and 5, showed no evidence of a leukocytic pleocytosis or of red cells, although CSF protein was noted to be elevated. Of perhaps greatest relevance, two successive PCR studies for herpes simplex virus in the CSF were reported as negative. Immediately following transfer to the Ben Taub General Hospital (day 7), a third lumbar puncture was performed, documenting for the first time a mild lymphocytic pleocytosis of the CSF. At this time, a positive PCR assay of the CSF for HSV type 1 was documented. An EEG performed shortly after transfer (see the "Test Results" menu) suggested the presence of a focal temporal lobe process. A follow-up MRI scan of the head demonstrated more characteristic findings of herpes simplex encephalitis affecting the temporal and frontal lobes:
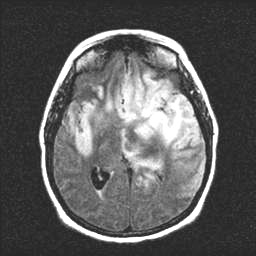
FLAIR
Based on the persistence of fever, and continued clinical and radiological progression despite early administration of acyclovir, the possibility of an acyclovir-resistant strain of herpes simplex virus was considered. Foscarnet was empirically added to the patient's regimen of acyclovir, with subsequent improvement in her fever and regaining of consciousness. The patient, however, sustained considerable neurologic injury from her infection, and at about six months from her initial presentation still exhibits disabling residual cognitive and motor impairment. She has been able to participate in rehabilitation and gait training, and continues to slowly improve.
The term "herpes" is derived from a Greek word meaning "to creep." The classically recognized presentation of HSV encephalitis involves the relatively abrupt onset of fever, focal signs, seizures, and/or deteriorating level of consciousness, and in such patients, the diagnosis is usually entertained early in the clinical course. However, many patients present with an initial prodromal phase of "creeping" progression, during which the disease may appear to smolder for a time before entering into a phase of rapid decline. In some of these patients, behavioral or psychiatric manifestations, amnestic symptoms, or language disturbance may be the presenting signs of illness, and the diagnosis of an early herpetic infection may be difficult to distinguish from other, more common, causes of psychiatric or neurologic dysfunction. Early in the course of herpetic infection of the CNS, the patient may not appear seriously ill. With the advent of PCR detection of viral DNA isolated from nucleated cells of the CSF, mild cases of HSV encephalitis and atypical presentations of HSV infections of the brain, have been documented (e.g., Fodor et al., 1998). Atypical presentations of HSV encephalitis has been associated with HSV type 2 infection, with immunosuppression (e.g., steroids or HIV disease), and with predominant involvement of the nondominant temporal lobe (Fodor et al., 1998; Jacobs, 1999).
The CSF examination in herpes simplex encephalitis is nearly always abnormal on the initial lumbar puncture, but may be minimally so. In one series, 4% of patients with confirmed HSV encephalitis had an initially normal cell count on the first LP, while 13% had an initially normal CSF protein (Kennedy, 1988). Other series have reported similar percentages of normal or near-normal initial CSF studies (reviewed in Fishman, 1992). PCR of CSF for HSV DNA is generally reported to have a high degree of sensitivity (95-97%) and specificity (99-100%) for active HSV infection of the CNS. However, in one recent series (Studahl et al., 1998), two of 10 patients with confirmed HSV encephalitis had initially negative PCR for HSV, with repeat studies becoming positive at times as late as seven days. Both of these patients had a short duration of disease (~2 days) at the time of the initial LP. Both EEG and MRI studies have been reported to be highly sensitive for detection of focal encephalitis; however, early in the course of illness (as was observed in this patient), these studies can also be negative. Given the documented efficacy and relatively modest toxicity of acyclovir, empiric treatment with antiviral therapy should be initiated on suspicion of HSV encephalitis, instead of waiting for confirmation of the diagnosis. We believe that continued empiric antiviral therapy should be strongly considered in patients with clinically suspected HSV encephalitis even if the initial PCR studies of CSF are negative, particularly in those patients studied early in the course of illness.
The differential diagnosis for HSV encephalitis is broad, and includes most CNS processes capable of producing fever, encephalopathy and focal neurological deficits. Of note in this case, titers for St. Louis encephalitis were reported as a borderline positive result, a finding that we consider to be of marginal specificity. Few cases of St. Louis encephalitis were reported in the Greater Houston area during the year of the patient's presentation, and the unusually dry weather at this time had kept mosquito populations low. In this patient, the focal abnormalities noted on the EEG and MRI following transfer, and the presence of an alternative diagnosis, rendered an arboviral encephalitis unlikely. The patient was not believed to be clinically immunosuppressed, and screens for HIV infection as well as specific assays for several opportunistic infections were negative, rendering these diagnoses unlikely. Likewise, screens for active inflammatory conditions such as systemic lupus were unremarkable. Normal clinical cardiovascular examination and echocardiography, together with negative blood cultures, were felt to exclude bacterial endocarditis, which can also mimic the fever, focal deficits, and alteration of mental status seen in patients with HSV encephalitis. A blood smear for malaria was negative, and the patient had no reported history of this condition. Finally, no evidence for septic cerebral venous thrombosis was found on either the initial or follow-up MRI studies.
Nucleoside analogs, such as acyclovir, have long been the mainstay of treatment in cases of suspected HSV encephalitis. Acyclovir is initially monophosphorylated by viral thymidine kinase (for which it has particular affinity), and subsequently converted into di- and triphosphate forms by cellular guanylate kinase and other cellular enzymes. The resultant acyclovir triphosphate, at least in vitro, competes with and also inactivates the viral DNA polymerase, and may also incorporate into the viral DNA itself. Acyclovir is given intravenously, typically 10-15 mg/kg every eight hours in the case of suspected HSV encephalitis, and continued for at least 14 days (e.g., Keating, 1999). Adverse effects include hypersensitivity, and renal tubular dysfunction, especially in the setting of dehydration or excessive dosage. Acyclovir has documented efficacy in the treatment of HSV encephalitis, reducing mortality and increasing the percentage of patients with little or no residual impairment (e.g., Skoldenberg et al., 1984; Whitley et al., 1986). However, even with early acyclovir treatment, persistent neurologic deficits frequently occur (McGrath et al., 1997), and disease may clinically progress even with early initiation of therapy.
Apparent failure of acyclovir treatment can occur following delay of therapy, early termination of therapy, or in the situation of catastrophically progressing disease. However, in this patient, acyclovir was initiated early in the course of disease, and fever and clinical progression continued despite maintenance of this regimen.Apparent failure of treatment can also occur as a result of evolving mass effect from edema or hemorrhage, seizure activity precipitated by persisting irritation, or an adverse immune-inflammatory response to the initial herpesvirus infection. In this patient, steroid treatment had been initiated early in the course of therapy, and breakthrough seizures were clinically and electrographically controlled with anticonvulsant medications. These observations suggested the possibilities of viral resistance to acyclovir, or of unfavorable drug pharmacokinetics, as causes of poor clinical response to therapy, and another antiviral agent, foscarnet, was empirically added to the patient's regimen.
Resistance of HSV to acyclovir has typically been documented in immunocompromised patients on viral suppression regimens, or in patients receiving extended therapy for ocular or chronic mucosal infections (e.g., Chakrabarti et al., 2000). Its incidence in the community of non-immunocompromised and previously untreated hosts is likely to be low but has not been convincingly documented. Resistance may result from mutations in the viral thymidine kinase that affect its expression, affinity for acyclovir, or phosphorylation function. Alternatively and much less likely, resistance can emerge from alterations in the viral DNA polymerase rendering it less susceptible to acyclovir triphosphate inhibition. Foscarnet (phosphonoformic acid), a pyrophosphate analog, inhibits viral DNA polymerases at the pyrophosphate binding site. It does not require phosphorylation by thymidine kinase for its activity, and therefore represents an alternative means of inhibiting viral replication, especially in thymidine kinase mutant strains. In addition, additive effects of foscarnet and thymidine kinase-activated nucleoside analogs on viral replication have been documented in vitro. The use of foscarnet in HSV encephalitis has recently been reported anecdotally (e.g., Plantinga and Vanneste, 2001). This case represents an additional report of apparent response to foscarnet in HSV encephalitis with documented progression during acyclovir treatment. The nephrotoxicity of foscarnet is considerable, making it a second-line agent for most viral infections. The use of other drugs that inhibit HSV replication, such as cidofovir, has not been reported to date in the treatment of herpes simplex encephalitis with apparently poor clinical response to acyclovir.
We would like to emphasize that the possibility of HSV resistance to acyclovir in this patient is not proven. The PCR assay used in this case detects only markers flanking the viral thymidine kinase gene, and did not allow recovery of this gene or others for sequencing. A brain biopsy to recover virus for culture and DNA sequencing, and to measure tissue drug levels, was felt to be unlikely to offer clinical benefit beyond that of empiric treatment in this patient with rapidly progressing disease. Addition or substitution of other antiviral agents in patients with HSV encephalitis and apparent failure to respond to acyclovir is based on anecdotal evidence (e.g., Plantinga and Vanneste, 2001). The decision as to whether an apparent failure of therapy may result from viral resistance or unfavorable pharmacokinetics remains a clinical judgement, as in vitro drug susceptibility assays poorly predict clinical response to treatment (e.g., Chakrabarti et al., 2000), and rapid sequencing of the herpesviral thymidine kinase and DNA polymerase genes is not generally available. This case illustrates the difficulties involved in current diagnosis and management of HSV infection of the CNS, and the need for further research to enhance decision-making and improve outcomes in this devastating disorder.
Email comments: