Aortic Constriction/Coarctation and Cardiac Stress
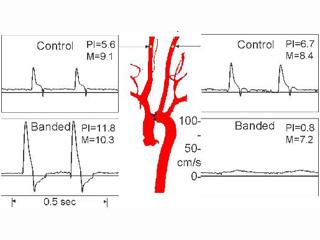
Because baseline cardiac function can be insufficiently revealing, we have developed a calibrated method of inducing increased left ventricular work in mice that has allowed us to evaluate the effects of hemodynamic stress on various transgenic and gene deletion models [Kurrelmeyer et al, 2000]. Mice are taken at 8-12 weeks of age 20-25 g body weight and are subjected to partial occlusion of the aorta utilizing a technique developed in the laboratory. To create the occlusion, a 6-0 suture is tied twice around a blunt 3mm segment of a 27 gauge needle, which is positioned adjacent to the aorta between the r. innominate and left carotid arteries, then the needle is removed after placement of the ligature. The degree of constriction is controlled by measuring peak flow velocity in each of the carotid arteries before and after constriction.
Depending on the experiment, animals are subjected to degrees of constriction resulting in right to left peak velocity of 5-10. Mice are monitored again at two days to assure that the load is remaining constant. Using this method, we have had considerable success in developing constant amounts of left ventricular hypertrophy in a cohort of mice. This method has been successful in showing stress related changes [Kurrelmeyer et al, 2000]. Sham animals are generated by following the entire surgical procedure without constricting the ligature. The constrictors are placed on the aorta at 14 days which corresponds to the formation of mature scar in the mouse [Michael et al, 1995; Michael et al, 1999].
Figure: Carotid artery velocity signals from a normal mouse before and after placement of a constricting band around aortic arch. The band creates pressure overload cardiac hypertrophy, and analysis of the differences in the carotid flow pulsatility (PI) index taken at surgery or at one day post-op can be used to predict the degree of hypertrophy after one week. It is interesting that the mean velocities (M) change very little after banding.
Serial Cardiac Function in Aging and Obese Mice
In response to new research initiatives, we have developed the expertise to serially monitor cardiovascular function in aging and obese mice which require more post-operative and peri-procedural care to allow serial survival monitoring.
Functional studies serially assess cardiac function (Medrano et al, 2016) in aged mice treated either with various agents or with vehicle (control) for extended time (one to three months, but longer is possible if needed). At time zero, non-invasive measurements of “baseline” cardiac function and structure using echo and Doppler are performed under 1% isofluorane anesthesia. Then mice are randomly allocated to treatment and control groups. Animals undergo by 2D echocardiography (Vevo 770 RMV-707B 30-MHz probe, VisualSonics) and Doppler (DSPW, 10-MHz) measurements (Cieslik et al, 2011; Haudek et al, 2010) every month to identify potential changes in cardiac function. Quantitation of LV function and structure are done with two-dimensionally guided M-mode images from parasternal short-axis views at the midpapillary level. We use left atrial volume as a structural indicator of elevated LV end diastolic pressures. Left atrial dimensions are collected to obtain the chamber volume using prolate ellipse: LA Volume = (4π x D1 x D2 x L)/(3x2x2x2). Cardiac aging is characterized by measuring systolic parameters (LVID, LVPW thickness, LV Volume, Cardiac Output, Peak Aortic Velocity) and diastolic parameters (E-Peak Velocity, E/A Peak Velocity ratio, Isovolumic Relaxation Time and LA Volume) correcting these for heart rate. The Doppler mitral flow velocity envelope is analyzed using a parametric model that allows discrimination of stiffness from viscoelastic elements. This approach allowed us to quantitate the fibrosis contribution in the aged mouse heart. It is essential to note that each mouse will serve as its own control.
First, we assessed cardiac function in the aging mouse heart (Cieslik et al, 2011; Trial et al, 2017), then we started to examine heart function in response to various treatments where each animal serves as its own control (Cieslik et al, 2013; Cieslik et al, 2018).







